The term lipid is applied to those fatty, oily and waxy substances of animal or vegetable origin. In physiologic fluids and in tissues, most lipid molecules are present in combination with protein referred to as lipoproteins. This combination promotes solubility of the lipids in aqueous medium.
The lipids include sterols, Vitamin A, D, E and K, bile pigments, waxes, carotene and related dietary pigments, as well as the fatty acids, triglycerides and phosphatides.
In human serum, the principal lipids found are free and esterified cholesterol, phospholipids, triglycerides (neutral fats) and non–esterified fatty acids (NEFA and UFA). Although NEFA is present in small quantity, it represents one of the most active metabolic constituents found in serum. The solubility of lipids when in serum is due to their association with the more polar ones such as phospholipids and then combining with protein. It is this way that triglycerides derived from intestinal absorption of fat are transported in blood as (1) chylomicrons, and triglycerides derived from the liver are delivered as (2) low density lipoproteins.
The characteristic feature of lipids are:
1. The substance must be insoluble in water.
2. It must be soluble in lipid solvents like ether, chloroform and benzene.
3. The substance must be actually or potentially an ester of fatty acids.
4. The substance must be utilized by living organism.
Functions of lipids:
1. As membrane structural components.
2. As intracellular storage depots of metabolic fuel.
3. As transport form of metabolic fuel.
4. As protective form of the cell walls of many bacteria, of the leaves of higher plants, of exoskeleton of insects and the skin of vertebrates.
5. As regulatory substances.
6. As transport form of some neurotransmitters.
7. As receptors in nerve ending membrane.
8. As determinants of immunological specificity.
9. As enzyme cofactor.
Classification of lipids:
I. Simple lipids – esters of fatty acids with various alcohols.
A. Neutral fats – esters of fatty acids with glycerol
e.g. triglycerides
B. Waxes – esters of fatty acids with higher alcohols other than glycerols.
e.g. cholesterols
II. Compound or conjugated lipids – esters of fatty acids containing groups in addition to a fatty acid and an alcohol.
A. Phospholipids – fats containing in addition to a fatty acid and glycerol, a phosphoric acid residue, nitrogen containing bases and other constituents.
1. Lecithin (phosphatidyl choline) – one fatty acid esterified to glycerol is replaced by phosphoric acid and choline.
2. Cephalin (phosphatidyl ethanolamine) – serine or ethanolamine is present in place of serine.
3. Sphingomyelin – has no glycerol present
4. Cardiolipin
5. Plasmalogen
B. Glycolipids or cerebrosides – contain a high molecular weight fatty acid, a carbohydrate (glucose and galactose) with hydrogen but no glycerol (sphingosine); it is found in nerve tissue but not in human serum to any extent.
1. Sulfatides – sulfate derivatives of the galactosyl residue in cerebrosides.
2. Gangliosides – glycolipids occurring in brain which contain N–acetyl neuramino acid (sialic acid).
C. Others – include aminolipids and sulfolipids.
e.g. lipoproteins
III. Derived lipids – substances derived from the above groups by hydrolysis; e.g. fatty acids (saturated and unsaturated), glycerol, sterols, other alcohols, fatty aldehydes and proteins of lipoproteins.
Lipid metabolism:
Lipolysis is the breakdown or hydrolysis of the substance such as triglycerides into fatty acids and glycerol occurs through the action of the lipase. This reaction is important before further catabolism can proceed. Free fatty acid (FFA) derived from this breakdown is released into the serum as an albumin FFA complex. The uptake into tissue cells and subsequent oxidation into acetyl Co–A which can be further oxidized to carbon dioxide and water via the Kreb’s or tricarboxylic acid cycle take place. This is summarily described in the following reaction:
In a backward reaction, lipogenesis can take place. In this process, glucose and intermediate products like pyruvate and acetyl Co–A are synthesized into fat.
These process involved in lipid metabolism all takes place in the liver.
METHODS OF LIPID DETERMINATION
I. Total lipids – Gravimetric method of Pernokiz, Freeland and Kraus.
Principle:
Lipids are extracted from serum with a mixture of alcohol and ether. The extract is evaporated to dryness and the residue is re–extracted with hot petroleum ether. The petroleum extract is then evaporated and the residue is accurately weighed.
Clinical significance:
Increased – after a fat meal
Decreased – in steatorrhea (excessive loss of fats in the feces) and other malabsorption syndromes.
II. Triglycerides – these are esters of glycerol and usually, three different fatty acids. They constitute about 95% of adipose tissue and are the main form of lipid in man. They are transported mostly in the form of chylomicrons and VLDL.
Three basic methods for determination of triglycerides:
1. Enzymatic
2. Colorimetric – glycerol is oxidized to formaldehyde
3. Fluorometric – measures formed formaldehyde
Methodologies for triglyceride determinations can be grouped into 3 general categories:
1. Isopropanol extraction followed by solid phase adsorption
2. Enzymatic methods
3. Partition of serum lipid between two liquid.
The latter method is highly selective for triglycerides, is not affected by triglycerol and does not require sample blank correction except for highly icteric specimen.
Colorimetric methods for triglycerides determination:
1. Van Handel and Zilversmit
a. Extraction of serum lipids with Folch’s reagent (chloroform–ethanol mixture) and removal of phospholipids with an adsorbent (zeolite or alumina of florisil)
b. Triglycerides are then saponized with alcoholic KOH which results in the hydrolysis of triacylglycerols (TAG) to glycerol and fatty acids.
c. Glycerol is then oxidized with periodic acid to produce formaldehyde which is then treated with a sulfuric acid solution of chromotropic acid (H2SO4) to form a blue colored compound.
The reaction is summarized as follows:
2. Hantzch condensation reaction
a. Formaldehyde is made to react with diacetylacetone and ammonium ions producing 3, 5 diacetyl–1, 4 dihydrolutidine, a yellow fluorescent compound with maximal absorbance at 412 nm. The lutidine derivative which forms fluorescence forms the basis of fluorometric procedure especially suitable for automated analysis.
3. Enzymatic method
a. Breakdown of triglycerides by lipase (LPS)
b. Phosphorylation of glycerol catalyzed by glycerol kinase (GK)
· These method uses hydrazine which complexes with DHAP to shift the equilibrium strongly to the right, driving the reaction to completion. The decrease in absorbance at 340 nm is equal to the amount of glycerol present in the specimen.
Other enzymatic method generate hydrogen peroxide from glycerol–3–phosphate by using L–glycerophosphate oxidase (LGPO):
Clinical significance:
(1) Increased:
Atherosclerosis
Hypothyroidism
Coronary artery disease
Hypertension
Diabetes mellitus
Atheroma
(2) Decreased: No significance
Precautions in triglyceride determination:
1. Patient must fast for 12–14 hours since the level of TAG reaches its peak 2–6 hours after eating.
2. Plasma has a slightly higher level the serum because during the clotting process, the chylomicrons and lipoprotein become trapped in the clot with subsequent lipolysis also occurring.
3. Fluorides and oxalates cause interference in TAG determination.
4. Glucose and phospholipids interfere with TAG determination because the oxidation step of glycerol to formaldehyde is not specific for glycerol.
5. Interfering substances maybe removed with the use of adsorbents during the extraction step, like zeolite (magnesium aluminum silicate), salicic acid or alumina.
Correlation of serum appearance with triglyceride level:
III. Total Fatty Acids
Total fatty acids are derived from the laboratory hydrolysis of the serum phospholipids, triglyceride and cholesterol esters.
Forms of fatty acids:
1. Esterified fatty acids – 95%
2. Free fatty acids – 5%
A. Total Fatty Acids (Man and Gildea)
Principle:
Serum is extracted by refluxing with alcohol–ether and the extract is saponified with KOH. The fatty acids are precipitated, separated and titrated with standard KOH.
B. Fatty Acid Esters
Principle:
It is based on the formation of hydroxamates from the reaction of the esters with hydroxylamine in alkaline solution and the formation of a colored complex upon the addition of ferric chloride.
Note:
Since only 3–5% of the fatty acids in serum are unesterified, this procedure to determine the esterified fatty acids is often satisfactory as measure of the total fatty acids of serum.
C. Free Fatty Acids
Free fatty acids (FFA) or Non–esterified fatty acid (NEFA) are transported in the plasma complexed with albumin, to the liver, muscle and other tissues. During the fasting state, they are derived mainly from the hydrolysis of TAG in adipose tissues and to a smaller degree, from the circulating triglyceride rich lipoproteins (chylomicrons and VLDL).
Principle:
FFA are determined by titrating with alkali after direct extraction with a non–polar solvents.
Clinical Significance:
Diabetes Mellitus
After injection of epinephrine
After a fatty meal
Lipolytic mobilization is
promoted by:
Growth hormone,
Glucagon,
Adrenocorticosteroid
Pituitary Hormone
retarded by:
Epinephrine
Insulin
IV. Cholesterol
The second most abundant lipid in man which is an unsaturated steroid alcohol whose structure is based on the cyclopentanoperhydrophenantrene (CPPP) nucleus.
This is fat–like substance found in blood, bile and brain tissue. It is a precursor of bile acids and a number of steroid hormones. The distribution of cholesterol is even between plasma and the red blood cells. About 60–70% of cholesterol in the serum is in the form of cholesterol esters while that of the red blood cells and tissue cells are made up mostly of free cholesterol.
The liver is the main site of synthesis, but the skin, adrenals, gonads, intestine and even the aorta can carry out the biosynthesis. Cholesterol is excreted by conversion to bile acids and neutral sterols in the liver through the urine. Its absorption is enhanced by bile and pancreatic juice.
A. Total cholesterol
The following classification of procedures is based primarily on the nature of preliminary treatment of the specimen.
1. Single step procedure (Direct procedure)
In these methods, the colorimetric reaction is performed directly on serum or plasma.
Serious errors are liable to occur due to:
a. Presence of proteins
b. Interferences of non–specific chromogen including bilirubin
c. Errors due to difference in chromogenecity of cholesterol and cholesterol esters.
d. Results are not accurate unless it is done with internal standardization.
Examples of this method are:
a. Pearson, Stern and MacGavack
b. Zlatkiss, Zak and Boyle
c. Wybenga, et. al.
2. Two step procedure (Extraction procedure)
These methods introduce an extraction step, primarily to remove proteins prior to color development. Certain solvents or solvent mixtures that can dissolve small amounts of water and cause proteins to precipitate are necessary for a complete extraction of lipid.
Following extraction of the lipids, the extracting solvent is evaporated and the color reaction is applied to the lipid residue.
The method is still subject to errors caused by non–specific chromogens like bilirubin and unequal chromogenecity of the cholesterol and cholesterol esters.
e.g. Carr–Drekter method
3. Three step procedure (Extraction and Hydrolysis procedure)
In this approach, cholesterol and cholesterol esters are extracted and the latter are hydrolyzed or saponified before color development. Such procedures eliminate interference from protein and the problem if unequal rates of color development by the free and ester forms of cholesterol. The saponification step also tends to destroy some non–specific chromogens.
e.g. Abell–Kendall, Levie, Brodie and Kendall
4. Four step procedure (Procedure involving Extraction, Hydrolysis and Precipitation)
These methods are complicated and tedious but reliable. The steps of the procedure are as follows:
a. The cholesterol is extracted.
b. The total cholesterol is then further purified by isolation as the digitonide.
c. The digitonide is decomposed by saponification, which again frees the cholesterol.
d. Treatment of the free cholesterol with a color reagent to produce the necessary color.
By introduction of the digitonin step, the effect of non–specific chromogens is considerably reduced or eliminated.
e.g. Schoenheimer and Sperry method
Hydrolysis or saponification of cholesterol
esters is done to
a. Permit digitonide precipitation of all the cholesterol.
b. Obtain more complete extractability into certain solvents, e.g. petroluem ether.
c. Allow a color reaction to be made with one form of cholesterol only.
Free cholesterol and cholesterol esters yield different color equivalents. Most widely used technique for saponification or hydrolysis is the treatment of cholesterol with warm KOH.
5. Photometric methods – cholesterol reacts with a variety of strong acidic substances to give colored products. Cholesterol as well as other steroids give an intense color when treated with acid reagent.
a. Lieberman–Burchard
Solution of cholesterol in acetic anhydride when treated with sulfuric acid produces a display of colors from red, violet to blue green.
Burchard applied the reaction to cholesterol in chloroform and a blue–green color was produced by passing the red color.
Lieberman–Burchard reaction – cholesterol in chloroform is treated with acetic anhydride and concentrated sulfuric to produce a green color. Some investigators thought that the acetic acid anhydride acts as a diluent, as it can be replaced by other reagents like acetic acid, ethyl acetate and butanol.
Procedures using Lieberman–Burchard reaction are those described by:
(1) Bloor
An aliquot of the ether–alcohol extract is heated to dryness. Cholesterol is extracted from the residue with chloroform and this is reacted with a mixture
of concentrated sulfuric acid and acetic anhydride producing a green color that is determined colorimetrically.
Reagents:
Alcohol–ether mixture consisting of:
Alcohol – 3 parts – precipitate proteins
Ether – 1 part – extract lipids
Chloroform – extracts cholesterol from the residue
Concentrated sulfuric acid and acetic anhydride –
color developer
(2) Ferro–Ham method
The color developer is added directly to the serum and the mixture is read immediately. This method is less accurate but time–saving.
Color developing mixture consists of:
Glacial acetic acid – 20 cc
Acetic anhydride – 30 cc
Concentrated sulfuric acid – 5 cc
Prepare just before use.
(3) Schoenheimer and Sperry
(4) Drekter, Kingsley and Schaffort
(5) Abel, et. al.
b. Salkowski’s reaction – the reaction differs from Lieberman–Burchard reaction that only concentrated sulfuric acid is used. The acid is added to an equal volume of a solution of cholesterol in chloroform. After shaking, the chloroform layer becomes red purple and the acid layer presents a deep green fluorescence.
The method was never popular. Its success depends on low temperature and vigorous exclusion of moisture.
c. Techugaeff reaction – Techugaeff described the formation of red color when cholesterol reacts with acetyl chloride and zinc chloride in glacial acetic acid.
Cholesterol, cholesterol didgitonide and cholesterol esters all give the red color with an adsorption peak of 528 nm.
d. Method employing the ferric chloride–sulfuric acid reaction – the reaction is four to five times more sensitive than the Lieberman–Burchard reaction. The adsorption peak of the color produced is at 560 nm and is the same for free cholesterol and its esters.
Zlatkiss, Zak and Boyle applied the reaction directly to serum without any preliminary extraction of the cholesterol.
e. p–toluenesulfonic acid reaction – Pearson, Stern and McGavack described a method in which the color developed directly in serum without extraction.
Pearson, Stern and McGavack method (Direct method)
p–toluenesulfonic acid liberates cholesterol
from lipoproteins for direct reaction with
sulfuric acid in the presence of acetic
anhydride and acetic acid.
6. Enzymatic method
a. Hydrolysis of the cholesterol esters by cholesterol esterase (CE):
b. Oxidation of cholesterol with cholesterol oxidase (CO) to form hydrogen peroxide:
c. Oxidation of a quinoneimine dye by H2O2 in peroxidase (P) – catalyzed reaction to produce the colored end product:
7. Polarographic oxygen electrode – this method measures the decrease in oxygen tension during the reaction as cholesterol is oxidized to hydrogen peroxide in the enzymatic method. However, this require the use of a different type of instrument and the additional burden of maintaining the electrode and its membrane.
B. Cholesterol esters
0.5% digitonide is added to an alcohol–ether extract which precipitates the freecholesterol as digitonide. Petroleum ether is used to extract the esters. An aliquot of the extract is evaporated to dryness and the cholesterol esters are extracted with chloroform and the color is developed by Lieberman–Burchard reaction by addition of acetic anhydride and sulfuric acid.
Interferences in cholesterol determination:
1. Increased cholesterol values:
a. Presence of hemolysis since it:
(1) Contributes extra cholesterol from the RBC.
(2) Introduces an excess amount of iron from hemoglobin.
(3) Produces a red brown reaction product that will increase the density of specimen.
b. Prolonged incubation time of color developer.
c. Presence of chromogenic substances like hemoglobin and bilirubin (for every 1 mg% of bilirubin above the normal, there is a corresponding 5–6 mg% increase in cholesterol)
2. Decreased cholesterol values:
a. Introduction of water causes rapid fading of color.
b. Extreme exposure to light.
c. Presence of metallic contamination.
d. Anticoagulated blood, since anticoagulants may dilute the plasma with cell water by increasing the osmotic pressure thereby producing low results, thus serum is the preferred specimen.
Factors influencing cholesterol values:
Clinical significance of total cholesterol values:
1. Increased values in:
a. Obstructive jaundice
b. Hypothyroidism
c. Lipoidosis
d. Uncontrolled diabetes
e. Xanthomatosis
f. Nephrotic syndrome
g. Lipemia
h. Pregnancy
i. Aplastic Anemia
j. Familial Hypercholesterolemia
k. Multiple Sclerosis
2. Decreased values in:
a. Hyperthyroidism
b. Pernicious anemia
c. Gaucher’s disease
d. Severe Infections
e. Epilepsy
The most abundant form of lipids in man which are esters of glycerol that contain two fatty acyl groups and phosphatidic acid. Most are transported by HDL.
Plasma phospholipids are composed of approximately 75–80% lecithin, 15–20% sphingomyelin and about 5% cephalins. Lecithin and cephalin are phospholipids which are structural materials required by all types of living cell.
Principle of determination:
The organic portion of the phospholipids is oxidized by heating with a strong oxidizing agent (perchloric acid) and the phosphorous is converted to phosphate. Phosphate is determined by a reaction in which it is converted into a blue phosphomolybdate by a reducing agent. Phospholipid may either be precipitated with the protein or extracted with alcohol ether. If only the lipid phosphorous is to be determined along with the acid–soluble (inorganic phosphorous), the trichloroacetic precipitation maybe used. If other lipids such as cholesterol or fatty acids are to be determined as well, it maybe more convenient to use alcohol–ether extract since the other lipid can be determined on further aliquots of the same extract. Other methods for inorganic phosphate may be used for the final colorimetric determination.
Clinical significance: Increased in atherosclerosis
VI. Lipoproteins
Lipoproteins are macromolecular complexes that serve as transport vehicles of insoluble lipids in the plasma. In general, they consist of a lipid core of non–polar TAG and cholesteryl ester surrounded by more polar phospholipid, cholesterol and apoproteins.
Four major lipoproteins classes can be identified based on:
a. Particle size
b. Chemical composition
c. Flotation characteristics
d. Physicochemical properties
e. Electrophoretic mobility
Their density increases as:
a. Protein content increases
b. Lipid content falls
c. Size of particle becomes smaller
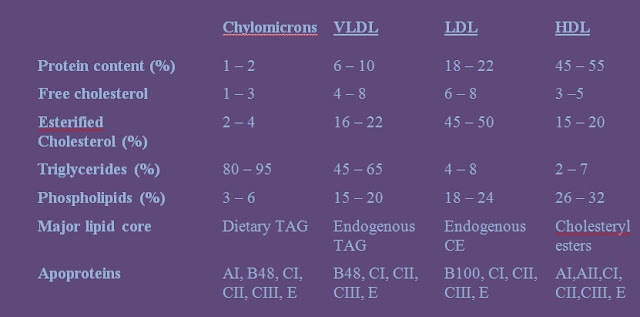
Chemical composition of lipoproteins:
The four major lipoproteins:
a. Produced by intestine.
b. The lightest of all lipoproteins due to its very high content of triglycerides of exogenous (dietary) origin.
c. Accounted for “milky” plasma after meals (due to high chylomicrons content).
d. Present as a floating creamy layer when plasma is left undisturbed for several hours.
e. Scatters light due to its very large complex.
f. Major apoproteins: CI, CII and B48
a. Synthesized in the liver from chylomicrons remnants.
b. Transports TAG of endogenous origin.
c. Smaller than chylomicrons and have lower lipid – protein ration thus floats at a higher density.
d. Excessive amounts make plasma turbid.
e. Like chylomicrons, they are large enough to scatter light.
f. Catabolism gives rise to intermediate density lipoproteins (IDL).
g. Major apoproteins: B100, ApoE, ApoC
a. Derived mainly from VLDL.
b. Constitute about 50% of the total lipoproteins mass in plasma.
c. Cholesteryl esters account for about half of LDL mass.
d. Do not scatter light nor alter the clarity of plasma.
e. Supplies cholesterol to extrahepatic cells like adrenal cortical, muscle and renal cells.
f. Levels increase after an overnight fast.
g. Associated with greater risk of developing atherosclerosis.
h. Major apoproteins: B100
a. Produced by the liver and intestine.
b. Referred to as the “good cholesterol.”
c. Facilitates the catabolism of VLDL and chylomicrons.
d. More proportion of protein than lipids.
e. Esterogen increases its concentration while testosterone decreases it.
f. Increased levels are associated with decreased risk of developing atherosclerosis.
g. Provides protection against coronory artery disease (CAD).
h. Major apoprotein: AI and AII
Other tests for lipids:
1. Fat tolerance test – to detect derangement of lipid metabolism and for assessing susceptibility to atherosclerosis and coronary heart disease.
Procedure:
a. Patient is allowed to fast overnight.
b. A fasting blood specimen is drawn.
c. A standard fat meal consisting of 100 ml of cream (approximately 40 g of fat) with cocoa and sugar for taste is given,
d. Succeeding blood specimens are drawn at intervals of 60, 120, 150, 180 and 210 minutes.
e. The total esterified fatty acids and visible lipemia are determined on each specimen.
f. Visible lipemia is determined turbidimetrically by diluting serum 2.5 times its volume with a 20% urea solution and reading the OD at 650 nm.
g. Maximum fatty acid concentration and turbidity appear at about 180 minutes in all subjects. Normal individuals will show an increase to about 17.5 mEq/L. In atherosclerosis, fatty acid will increase to about 23 mEq/L and will show a slower return to the fasting level.
2. Chylomicron count
Blood is drawn from the finger tip of a fasting patient. A test meal is then given consisting 30 cc heavy cream per kg. body weight but not greater than 120 cc. Nothing else is given by mouth during the five hours of the study. After the fasting meal, a capillary blood sample is taken at hourly intervals. Specimens are centrifuged and the serum is placed on a glass slide. It is examined under dark field technique and the number of the particles of visible fat is counted in a single plane of the oil immersion field.
Results:
The fat particles measure about 1 micron in diameter. A curve drawn to show the rise and fall of the count after a fatty meal is called a chylomicrograph. The peak of the curve in normal children is reached in 2 to 3 hours and average about 53 particles / field. In celiac diseases, the count increases more slowly and do not exceed an average maximum of 20 particles per field within 6 to 10 hours, almost all of the chylomicrons in the blood will have vanished if no further fat has been ingested.
Clinical significance:
Flatter graphs are observed in the chylomicron counts of individuals with celiac disease. This suggests that the absorption of particulate fat from and individual’s small intestine is defective in celiac disease. Similar impairment of absorption occurs in fibrocystic disease of the pancreas.
3. Examination of stool for fat
A representative sample of the patient’s stool is mixed with distilled water on a glass slide. Two drops of 95% ethanol is then added, mixed and 2 or 3 drops of Sudan III in 95% ethanol is subsequently added. Place a cover slip and examine under high dry lens.
To another stool mixture, add 2 or 3 drops of 36% acetic acid and mix to an even consistency. Add 2 or 3 drops of Sudan III solution, mix and cover with a cover slip. Gently heat the mixture over an alcohol burner until it starts to boil. Repeat the process quickly, two or three times to melt the fatty acid crystals. Examine under high power magnification. Use a standardized micrometer to measure the diameter of the fat globules.
Results:
The presence of neutral fat will be observed as yellow or pale orange refractile globules on the first slide. Normally, only few of any of these appear in the stool. On the second slide, stained fatty acid appears when warm as deep orange fat globules from which, as the preparation cools, spicules and soap forms, resembling the pinna of the ear, crystallize.
Stool with normal and 1+ amounts of fatty acids may show as many as 100 tiny globules per HPO with diameters between 1–4 micra. If the amount is 4+ there is clinical steatorrhea. A celiac syndrome is present if fat lost in stool is greater than normal compared to the amount ingested.
Clinical significance:
1. Hyperlipoproteinemia
Elevated plasma lipoproteins have been clinically important in the genesis of atherosclerosis and pancreatitis. Hyperlipoproteinemia is present in:
a. Any individual <20 years old with a total plasma cholesterol exceeding 200 mg/dl or with a TAG level > 140 mg/dl.
b. Any individual >20 years old with a total plasma cholesterol exceeding 250 mg/dl or with a TAG level > 200 mg/dl.
Frederickson–Levy Classification of Hyperlipoproteinemia (HLP):
This classification is based on laboratory findings or phenotypic expressions and not on pathophysiologic or genetic mechanisms.
a. Primary or Familial HLP:
Clinical manifestations:
(a) Xanthoma formation may occur in various regions of the body and lipids may even accumulate retina.
(b) Mild to severe pancreatitis as well as hepatomegaly, splenomegaly or both.
(c) Clinical manifestations are different for those with Apo CII deficiency. Most of these patients are 10 years of age or older and do not present with the eruptic xanthomas or hepatosplenomegaly. They do, however, have recurrent episodes of acute abdominal pain and pancreatitis after ingestion of a fatty meal.
Laboratory findings:
(a) Characteristic creamy layer forming at the surface of a fasting specimen.
(b) Triglyceride levels are markedly elevated, often requiring dilution before results are obtained.
(c) Despite pancreatitis, the serum amylase level may initially be falsely normal. Repeating the amylase test with serum diluted with normal saline solution may restore the expectedly elevated activity.
(a) Polygenic hypercholesterolemia or Severe Primary Cholesterolemia – patients exhibit a total cholesterol levels greater than 300 mg/dl with elevated LDL. Some cases exhibit tendinous xanthomas with increased risk of coronary artery disease (CAD).
(b) Familial hypercholesterolemia (FH) – inactive receptors either are not transported to the cell membrane or are unable to migrate along the cell membrane to the coated pits.
Homozygotes – display problems at early ages with tendon xanthomas and ECG indicating CAD.
Heterozygotes – diagnosed on the basis of their increased LDL levels along with nodular irregularities on the Achilles tendon (due to tendon xanthomas) and the extensor tendons, especially of the middle and ring fingers. Occur more frequently with increasing age and obesity. Their onset often follows a liver–related illness and apparently is not directly genetic in nature.
(c) Familial combined hyperlipidemia (FCHL) – patient is at risk of premature CAD and exhibit an elevated total VLDL, LDL cholesterol and triglycerides.
(a) With elevated plasma triglyceride and cholesterol levels.
(b) Reflects the presence of an abnormal form of VLDL, which shows beta–mobility on electrophoresis known as beta–VLDL (floating betalipoprotein).
(c) Biochemical abnormality has been characterized as a quantitative and qualitative abnormality of apoE.
(4) Type IV: Familial Hypertriglyceridemia – common disorder of lipid metabolism characterized by increased levels of plasma triglycerides and VLDL without any other specific clinical or biochemical features.
(a) With elevated levels of VLDL and chylomicrons, lipemia, retinalis, eruptive xanthoma and pancreatitis.
(b) Triglycerides and cholesterol levels are also elevated.
(c) With normal lipoprotein and hepatic lipase activities.
(d) Appears to be related to a deficiency in apoC–III
b. Secondary HLP:
Hepatobiliary Peripheral
related related
Acute viral hepatitis Myocardial infarction
Biliary obstruction Renal failure
Cirrhosis Gout
Acute pancreatitis Diabetes mellitus
2. Hypolipoproteinemia
a. Abetalipoproteinemia (Bassen–Kornzweig syndrome) – manifested by hypocholesterolemia and total absence of chylomicrons VLDL and LDL due to defective synthesis of Apo–B. It is characterized by fat malabsorption (steatorrhea), neuromuscular abnormalities (ataxia and hyporeflexia), retinitis pigmentosa, acanthocytosis and fat soluble vitamin deficiencies.
b. Tangier’s disease – a rare disorder in which cholesterol ester–laden cells accumulate in the reticuloendothelial tissue and characterized by the complete absence of the alpha lipoprotein band in electrophoresis. Clinically manifested by large orange yellow tonsils and adenoids, muscle weakness and atrophy and recurrent peripheral neuropathies with depressed tendon reflexes. The major problem with Tangier’s disease is its increased susceptibility to atherosclerosis.
c. Hypobetalipoproteinemia – this disorder is inherited as an autosomal dominant trait. The defect appears to be an inability to synthesize ApoB–100 and ApoB–48. With low total and LDL cholesterol levels and normal to low triglyceride levels, patients usually have a significant increase in life expectancy.
Methods of determining lipoprotein:
a. Ultracentrifugation – involves centrifugation at very high speed (up to 50,000 rpm) and gives result that are expressed in terms of Sf units, which is actually a flotation rate since lipoproteins tend to rise to the surface during centrifugation because they are less dense than the media used to dilute the serum. Sf unit stands for Svedberg unit of flotation. The Sf values have been used in the study of atherosclerosis and an increase in certain range of Sf values has been stated to be indicative of a tendency toward atherosclerosis.
b. Electrophoresis, either on paper or by starch zone method
This method distinguishes between two classes of lipoproteins, the alpha and beta lipoproteins. Normally, the alpha lipoproteins comprise 30–35% of the total lipoproteins and the beta fraction comprises the remaining 50–70%. In individual with atherosclerosis, the proportion of beta lipoproteins increases to 75–85% of the total.
In the starch zone method of electrophoresis, the lipoproteins measured in terms of their cholesterol content, are divided into three fractions, an alpha–1 fraction (25–35%), an alpha–2 fraction (8–12%) and a beta fraction (55–65%). In atherosclerosis, the beta fraction is increased up to 80% or higher.
c. Direct precipitation upon the addition of some high molecular weight compunds such as dextran sulfate, sulfonated amylopectin or heparin.
d. Immunologic reaction with specific antisera.
e. Low temperature fractionation
f. Methods for determining HDL–cholesterol values
ApoB containing lipoproteins (chylomicrons, VLDL, IDL, LDL, Lp(a) are removed by polyanion–divalent cation precipitation and HDL–cholesterol is analyzed directly in the supernatant. There are several methods but the most widely used is the heparin sulfate–manganese method.
g. Combined methods:
(1) Features:
(a) Measurement of plasma cholesterol, VLDL, LDL, HDL and triglyceride levels.
(b) Assessment of whether the patient has chylomicrons in the fasting state.
(c) Assessment of the presence or absence of beta–VLDL.
(2) Employs a combination of preparative ultracentrifugation, polyanion precipitation and electrophoresis.
(3) Plasma total cholesterol, HDL–cholesterol and triglycerides are determined directly while the other lipoprotein cholesterol concentration are calculated:
(a) LDL–cholesterol = (infranatant cholesterol ) – (HDL–cholesterol)
(b) VLDL–cholesterol = (total cholesterol) – (infranatant cholesterol)
(4) Lp(a) is found in the LDL–HDL density range and its contribution of about 5 mg/dl becomes included in the LDL–cholesterol measurement. Thus, a simplified procedure was described by Friedwald which does not require the ultracentrifugation:
(d) When concentrations are expressed in mg/dl,
(e) But a more accurate estimate of VLDL–cholesterol was suggested by Delong where:
(f)Limitation of the equation are as follows:
– must be performed on a fasting sample
– not suitable for use in which triglyceride concentration exceed 10.390mmol/L (400 mg/dl)
– not suitable for samples with chylomicrons or beta–VLDL
h. Standing plasma test
(1) An aliquot of plasma is placed in a 10 x 75 mm test tube and allowed to stand undisturbed overnight in a refrigerator at 4oC.
(2) Chylomicrons are detected visually as a “floating creamy layer.”
VII. Apoproteins
Apoproteins are protein units found in lipoproteins but not yet incorporated into their respective lipoprotein particles. When complexed into lipoprotein particles, they are referred to as apolipoproteins.
Types apoproteins:
1. Apoprotein AI is the major protein constituent of HDL. It functions as an activator of lecithin–cholesterol acyltransferase (LCAT), which catalyzes the formation of cholesterol esters. Apo AI in combination with Apo AII and Apo CI is responsible for the removal of free cholesterol from extrahepatic tissues.
a. Apo B–100 – a large 100–amino acid long structure synthesized in the liver and commonly found in plasma. It is found in VLDL and IDL but is predominantly found in LDL. It functions as the recognition site for the LDL molecule in its ability to bind to membranes.
b. Apo B–48 – a smaller 48–amino acid structure synthesized in the intestinal wall and hence tends to be associated with chylomicrons found in the lymph. As the chylomicrons are cleaved, Apo B–48 is also removed and not detected in appreciable amounts in fasting plasma except in patients with defects in clearing chylomicron remnants.
3. Apoprotein C functions in the activation of lipoprotein lipase (LPL), leading to the breakdown of triglycerides at the cellular level, with subsequent release of fatty acids to the cells for metabolism and storage. It is not detected in IDL or LDL, suggesting that HDL transfers its Apo C molecules to chylomicrons and VLDL and later picks them up again. It is synthesized in the liver.
b. Apo–CII – composed of 78 amino acid which is a required cofactor for exrtrahepatic clearance of VLDL and chylomicrons. Apo–CII also prevents these particles from binding to hepatic receptors, the first step in hepatic clearance.
4. Apoprotein D (thin–line peptide) – is a glycoprotein. Thought to be a transfer protein and is involved in the movement of cholesterol esters and triglyceride among the various lipoproteins.
5. Apoprotein E (arginine–rich peptide) – functions primarily as a marker for hepatic receptors. Synthesized in the liver, this is incorporated into HDL. HDL then transfers ApoE molecules VLDL and chylomicrons. As these particles are subsequently degraded to IDL and chylomicrons remnants. It has four polymorphic forms.

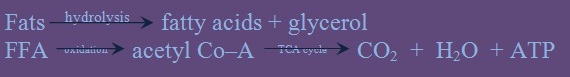










No comments:
Post a Comment