The
classical taxonomic classification divided the Protozoa into four groups:
a. Sarcodina
(amoebae)
b. Mastigophora
(flagellates)
c. Sporozoa
(most parasitic protozoa)
d. Infusoria
(ciliates)
This
taxonomy has been totally abandoned by the International Society of Protozoologists
based on modern morphological approaches such as biochemical pathways and molecular
phylogenetics (e.g., 18S rRNA sequences). The older hierarchical systems consisting
of the traditional “kingdom,” “phylum,” “class,” “subclass,” “super-order,” “order,”
has been replaced by a new vocabulary.
According
to this new schema, the Eukaryotes have been classified into six clusters or “Super
Groups,” namely:
a. Amoebozoa
b. Opisthokonta
c. Rhizaria
d. Archaeplastida
e. Chromalveolata
f. Excavata
The
three amoebae that are dealt within this article have been classified under two
Super Groups, Amoebozoa and Excavata, as follows:
a. Acanthamoeba
and Balamuthia are classified under Super Group Amoebozoa: Acanthamoebidae
b. Naegleria
fowleri under Super Group Excavata: Heterolobosia: Vahlkampfiidae
This
schema has been proposed as the basis for future revisions.
For
educational purposes, the classic phylogeny of the parasite was described here.
And unlike amoeba belonging to Phylum Sarcodina that requires host to survive,
parasites belonging in this group are free living (i.e., doesn’t require host
to survive).
NAEGLERIA
FOWLERI
Phylum
Percolozoa
Subphylum
Tetramitia
Order
Schizopyrenida
Family
Vahlkampfiidae
Genus
Naegleria
Naegleria
fowleri is an amphizoic amoeba, as it can survive in a free-living state in
water, soil, or in the host, which can be the human central nervous system
(CNS) and causes a disease known as Primary Amebic Meningoencephalitis (PAM)
thus its reputation as "brain-eating amoeba."
The
initial symptoms of PAM are indistinguishable from bacterial meningitis, while
the symptoms of GAE can mimic a brain abscess, encephalitis, or meningitis.
The
amoeboid stage is roughly cylindrical, typically around 20–40 μm in length.
They are traditionally considered lobose amoebae, but are not related to the
others, and unlike them, do not form true lobose pseudopods. Instead, they
advance by eruptive waves, where hemispherical bulges appear from the front
margin of the cell, which is clear. The flagellate stage is slightly smaller,
with two or four anterior flagella anterior to the feeding groove.

Naegleria
fowleri has been thought to infect the human body by entering the host through
the nose when water is splashed or forced into the nasal cavity. Infectivity
occurs first through attachment to the nasal mucosa, followed by locomotion
along the olfactory nerve and through the cribriform plate (which is more
porous in children and young adults) to reach the olfactory bulbs within the CNS.
Once Naegleria fowleri reaches the olfactory bulbs, it elicits a significant
immune response through activation of the innate immune system, including
macrophages and neutrophils. Naegleria fowleri enters the human body in the
trophozoite form. Structures on the surface of trophozoites known as food
cups enable the organism to ingest bacteria, fungi, and human tissue. In
addition to tissue destruction by the food cup, the pathogenicity of Naegleria
fowleri is dependent upon the release of cytolytic molecules, including acid
hydrolases, phospholipases, neuraminidases, and phospholipolytic enzymes that
play a role in host cell and nerve destruction. The combination of the
pathogenicity of Naegleria fowleri and the intense immune response resulting
from its presence results in significant nerve damage and subsequent CNS tissue
damage, which often result in death.

The
quickest way to diagnose Naegleria fowleri infection is by microscopic
examination of fresh, unfrozen, unrefrigerated cerebrospinal fluid (CSF).
Both
chlorinated and salt water significantly decrease the risk of Naegleria fowleri
infection due to its inability to survive in such environments. Thus, avoidance
of exposure to freshwater bodies such as lakes, rivers, and ponds, especially
during the summer months when the water temperature is higher.
ACANTHAMOEBA
SPP.
Phylum
Amoebozoa
Class
Conosea
Order
Centramoeba
Family
Acanthamoeba
Some
of the pathogenic species:
a. Acanthamoeba
castellanii
b. Acanthamoeba
culbertsoni
c. Acanthamoeba
polyphaga
d. Acanthamoeba
healyi
e. Acanthamoeba
divionensis
Diseases
caused by Acanthamoeba include keratitis and granulomatous amoebic encephalitis
(GAE).
Acanthamoeba
keratitis (AK) is associated with trauma to the cornea or contact-lens wear and
the use of amoeba–contaminated saline. Minor erosion of the corneal epithelium may
occur while wearing hard or soft contact lenses, and the subsequent use of contaminated
saline solution is the major risk factor for Acanthamoeba keratitis. AK is characterized
by inflammation of the cornea, severe ocular pain, and photophobia, a characteristic
360o or paracentral stromal ring infiltrate, recurrent breakdown of corneal
epithelium, and a corneal lesion refractory to the commonly used antibiotics. Typically,
only one eye is involved; however, bilateral keratitis has also been reported. It
is the MBP (mannose binding protein) that mediates the adhesion of the amoeba to
corneal epithelial cells and is central to the pathogenic potential of Acanthamoeba.
A
unique and characteristic feature of Acanthamoeba spp. is the presence of fine,
tapering, thorn-like acanthopodia that arise from the surface of the body.
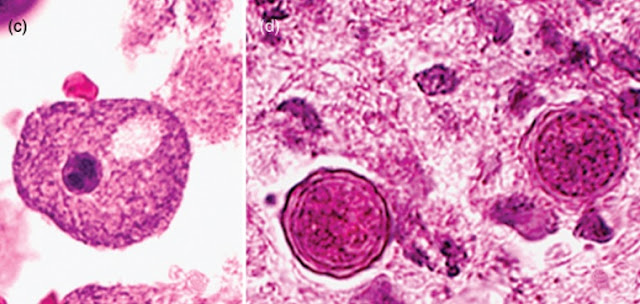
a. The
trophozoites range in size from 15 to 50mm depending upon the species. They are
uninucleate, and the nucleus has a centrally placed, large, densely staining nucleolus.
The cytoplasm is finely granular and contains numerous mitochondria, ribosomes,
food vacuoles, and a contractile vacuole. When food becomes scarce, or when it is
facing desiccation or other environmental stresses, the amoebae round up and encyst.
b. Cysts
are double-walled and range in size from 10 to 25mm. Cysts are uninucleate and possess
a centrally placed dense nucleolus. Upon return to favourable growth conditions,
the dormant amoeba is activated to leave the cyst by dislodging the operculum and
reverting to a trophic form
(1) The
outer cyst wall, the ectocyst, is wrinkled with folds and ripples and contains protein
and lipid.
(2) The
inner cyst wall, the endocyst, contains cellulose and hence is Periodic Acid Schiff
(PAS) positive. The endocyst varies in shape: it may best ellate, polygonal, oval,
or spherical.
(3) Pores
or ostioles that are covered by convex–concave plugs or opercula are present at
the junction of the ectocyst and the endocyst.
In either the trophic or the cyst stage these
organisms have a wide distribution in nature, and it is virtually impossible not
to isolate members of this genus from soil, water, and other samples.
Acanthamoeba
spp. Are ubiquitous and occur worldwide. They have been isolated from soil, fresh
and brackish waters, bottled mineral water, cooling towers of electric and nuclear
power plants, heating, ventilating and air conditioning units, humidifiers, Jacuzzi
tubs, hydrotherapy pools in hospitals, dental irrigation units, dialysis machines,
dust in the air, bacterial, fungal and mammalian cell cultures, contact-lens paraphernalia,
ear discharge, pulmonary secretions, swabs obtained from nasopharyngeal mucosa of
patients with respiratory complaints as well as of healthy individuals, maxillary
sinus, mandibular autografts, and stool samples. In addition, several Acanthamoeba
species have been isolated from the brain, lungs, skin, and cornea of infected individuals.
BALAMUTHIA
MANDRILLARIS
Balamuthia
mandrillaris is a free-living amoeba that is found in the soil and fresh water
and is associated with Granulomatous Amoebic Encephalitis (GAE), a “brain-eating”
disease both in humans and animals. Symptoms of granulomatous amebic
encephalitis begin gradually. Confusion, headache, and seizures are common.
People may have a low-grade fever, blurred vision, changes in personality, and
problems with speaking, coordination, or vision. One side of the body or face
may become paralyzed.
Balamuthia
mandrillaris may cause skin sores in addition to the symptoms above. Most
infected people die, usually 7 to 120 days after symptoms begin.
Possible
modes of transmission of Balamuthia include inhalation and inoculation through
broken skin.
Balamuthia
mandrillaris, like Acanthamoeba, has only two life-cycle stages, namely the vegetative
trophozoite and the dormant cyst.
a. The
trophozoite is pleomorphic and measures from 12 to 60 µm (mean of 30 µm). The
trophic amoebae are usually uninucleate, although binucleate forms are occasionally
seen. The nucleus contains a large, centrally placed, dense nucleolus;
occasionally, however, amoebae with two or three nucleolar bodies have been
seen, especially in infected tissues.
b. Cysts
are also uninucleate, are spherical, and range in size from 12 to 30 µm (mean
of 15 µm). Cysts, when examined with a light microscope, appear to be double
walled, the outer wall being wavy and the inner wall round, and pores are not
seen in the wall. Ultrastructurally, however, the cyst wall has three layers:
(1) an
outer thin and irregular ectocyst,
(2) an
inner thick endocyst, and
(3) a
middle amorphous fibrillar mesocyst.
In
general, Acanthamoeba spp. and Balamuthia are difficult to differentiate in
tissue sections by light microscopy because of their similar morphology.
However, they can be differentiated by immunofluorescence analysis of the
tissue sections using rabbit anti–Acanthamoeba or anti–B–mandrillaris sera.
While
Balamuthia and Naegleria share some similarities, Balamuthia is more difficult
to detect. This is due to its resemblance to histiocytes under the microscope and
unique culture requirements. Unlike Naegleria, Balamuthia cannot be grown on
agar because it only feeds on mammalian cells and other amoebas. Furthermore,
healthy individuals can be seropositive for Balamuthia antibodies due to the
amoeba’s pervasive presence in the environment, while those with GAE show low
titers. Additionally, cerebrospinal fluid analysis rarely demonstrates the
organism, and the time course for the appearance of lesions and the onset of
GAE is inconsistent. Balamuthia, unlike most of other free-living amoebae, does
not feed on Gram-negative bacteria and therefore the use of non-nutrient agar coated
with bacterial cultures has resulted to be ineffective for its growth.
These
amoebae were normally cultured on monolayers of African green monkey kidney
cells. Upon axenic cultivation, amoebae grew at various temperatures ranging
from 25°C to 37°C (optimal growth at 37°C) and remained viable for up to
several months, but they became smaller over time. In contrast, mammalian
cultures can be used persistently as feeder cells to culture Balamuthia amoebae
over longer periods, without any modifications in their general appearance. All
tested cell cultures, including human brain microvascular endothelial cells
(HBMEC), human lung fibroblasts, monkey kidney (E6) cells, and African green
monkey fibroblast-like kidney (Cos-7) cells, supported the growth of B.
mandrillaris.