Carbohydrates
are the major food supply and energy source of the body. Depending on dietary
habits, 50 – 90% consumed come from grain, starchy vegetables and legumes.
Typical items in this group include rice, wheat, corn and potatoes.
Carbohydrate
is the element in our food which supplies the energy for the body’s automatic
activity and for the performance of our daily tastes. Besides their usefulness
in supplying energy, carbohydrates play a vital part in the digestion,
assimilation and oxidation of protein and fat.
Despite the
major utilization of carbohydrate for energy, only a small amount of it is
stored in the body. The average adult reserve is about 370 grams stored chiefly
as liver and muscle glycogen. Since one gram of carbohydrate supplies 4 calories
or approximately half the average daily calorie needs. When the total calorie
intake exceeds the daily expenditure, the excess carbohydrate is readily
converted to fat and stored at adipose tissue.
The most
common disease related to carbohydrate metabolism is diabetes mellitus, which
is characterized by inability of the body to burn up (assimilate) glucose due
to deficient levels of active insulin. Deficiency of insulin results in
impaired metabolism, and increases in blood glucose concentration and secondary
changes in fat metabolism, leading eventually to ketosis and possible diabetic
coma. Other complications include hypercholesterolemia, atherosclerosis and
kidney disease. The condition of diabetes shows strong familial tendency; the
probability that an individual will develop diabetes is several times greater
when there is a family history of disease.
Early
recognition of diabetes will permit earlier management and perhaps delay or
minimize the complications of the disease. Overproduction or excess
administration of insulin causes a decrease in blood glucose level below
normal. In severe cases, the resulting extreme hypoglycemia is followed by
muscular spasms and loss of consciousness, known as insulin shock. Measurement
of blood glucose, therefore, assumes considerable significance and the clinical
laboratory must be prepared to furnish results rapidly and with high degree of
reliability.
The
determination of blood glucose is the procedure most frequently done in
hospital chemistry laboratory. In addition, many glucose determinations are
performed in clinics, independent laboratories and physician’s office as an aid
in the diagnosis and treatment of diabetes. The determination may be performed
on patients in the fasting state, in the post prandial state or in conjunction
with glucose tolerance test, in accordance with the physician’s request.
The normal
glucose concentration in blood is between 3.9 to 5.5 mmol / L (70 to 100 mg /
dl).
Chemistry of carbohydrates
The term
carbohydrate refers to hydrates of carbon and is derived from the observation
that the empirical formulas for these compounds contain approximately one
molecule of water per carbon atom. In more descriptive terminology, the
carbohydrates are defined as the aldehyde and ketone derivate of polyhydric
alcohols. The simplest carbohydrate is glycoaldehyde, the aldehyde derivatives
of ethylene glycol. The aldehyde and ketone derivates of glycerol are
glyceraldehyde (glycerose) and dihydroxyacetone, respectively.
The formula
for glucose may either be written in aldehyde or enol form. The presence of
double bond and a negative charge in the enol anion form make glucose an active
reducing substance and provide a basis for its analytical determination. Thus,
glucose in hot alkaline solution readily reduces metallic ions such as cupric
ions and the color change can be used as a presumptive indication for the
presence of glucose. Sugars capable of reducing cupric ions in alkaline
solution are commonly known as reducing sugars.
Monosaccharides
– are sugars containing, 3,4,5 and 6 or more carbon atoms. Aldehyde derivative
are called aldoses and ketone derivatives are called ketoses.
Typical
examples are the six–carbon sugars, glucose (an aldose) and fructose (a
ketose).
Disaccharides
are sugars formed by interaction of groups between two monosaccharides with
loss of a molecule of water. The chemical bond between saccharides always
involves the aldehydes or ketone group of one monosaccharides joined to the
other monosaccharides, either by latter’s aldehyde or ketone group (e.g.
sucrose) or by latter’s alcohol group (e.g. maltose). The linkage of an
aldehyde or ketone with alcohol is called glycosidic linkage.
Example of common dissacharides:
1.
Maltose
(glucose and glucose)
2.
Lactose
(glucose and galactose)
3.
Sucrose
(glucose and fructose)
Polysaccharides
result from the linkage of many monosaccharide units together.
Metabolism of carbohydrates
Starch and
glycogen are partially digested by the action of salivary amylase to form
intermediate dextrin and maltose. Amylase activity is inhibited at the acid pH
of the stomach. In the intestine, the pH is increased by alkaline pancreatic
juice and the amylase of the pancreas affects digestion of starch and glycogen
to maltose. The latter, along with any ingested lactose and sucrose, is split
by the dissacharides in the intestinal mucosa (maltase, lactase and sucrose) to
form the monosaccharide glucose, galactose and fructose.
Following
absorption into the portal vein, the hexoses are transported to the liver.
Depending on the needs of the body, the carbohydrates may be converted to keto
acids, amino acids and protein or converted to fat and stored as adipose
tissue.
Terms used to describe general processes in
carbohydrate metabolism:
1.
Glycogenesis
– conversion of glucose and other hexoses to glycogen by hepatic cells.
2.
Glycogenolysis
– breakdown of glycogen to form glucose and other immediate products.
3.
Gluconeogenesis
– formation of glucose from non – carbohydrate sources such as amino acids,
glycerol or fatty acids.
4.
Glycolysis
– conversion of glucose or other hexoses into lactate or pyruvate. S
Functions of carbohydrate:
1.
Providing
chemical energy to the body.
2.
Furnishing
part of the structural integrity of the cell.
3.
Determining
blood types.
Stability of glucose in body fluids:
1.
Glucose
is unstable when blood is permitted to clot and stand uncentrifuged at room
temperature and the average decrease is about 7% per hour.
2.
In
separated, unhemolyzed serum, the glucose concentration is stable up to 8 hours
at 25oC and up to 72 hours at 4oC.
3.
The
addition of sodium fluoride or iodoacetate will prevent glycolysis by
inhibiting phosphogyceraldehyde dehydrogenase and allow the glucose to be
stable for 24 hours at room temperature.
4.
Fluoride
ions inhibit urease activity and should therefore not be used for urea
determinations that require urease.
5.
Glucose
level is arterial blood is higher than in venous blood, while the capillary
blood approximates arterial blood. Glucose value in serum or plasma is about 10
– 15% higher than in whole blood.
6.
Plasma
of the whole blood fraction is the choice in glucose determination.
7.
Cerebrospinal
fluid glucose is about 2/3 of that of whole blood sugar.
Regulation of blood glucose concentration
In the
fasting state, the level of blood glucose is maintained by drawing upon the
glycogen stores of the liver and a slight amount may also be derived from the
kidneys. Both of these organs contain the specific enzyme, glucose–6–phosphatase
necessary for conversion of glucose–6–phosphate to glucose.
Skeletal
muscles, although it stores glycogen, is lacking in this enzyme and cannot
directly contribute to glucose in blood.
As blood
glycose level increases, usually by absorption of carbohydrates from
intestines, glycogenolyis is replaced by glycogenesis, whereby excess blood
glucose is converted into liver and muscle glycogen.
Hormones that regulate blood glucose
concentration
Insulin
Produced by
the beta cells of the pancreatic islets of Langerhans in response to an
elevated blood glucose level. It is the only hormone that lowers blood glucose.
It also alters the metabolic pathways of glucose metabolism by enhancing the
formation of glycogen, fat and proteins.
Glucagon
Produced by
the alpha cells of the pancreatic islet of Langerhans in response to a low
blood glucose level. It is the principal hormone for producing a rapid increase
in the concentration of glucose in the blood. It does so by stimulating hepatic
glycogenlysis and gluconeogenesis but has no effect on muscle glycogen.
Somatostatin
Produced by
the delta cells of the pancreatic islet of Langerhans and inhibits secretion of
insulin and glucagon, thereby modulating their reciprocating action.
Somatostatin only has minor effect on the blood glucose concentration.
ACTH
Adrenocorticotropic
hormone (ACTH), also called corticotropin, is a small polypeptide secreted by
the anterior pituitary. Like GH, it increases the concentration of blood
glucose because of its antagonistic action towards insulin.
Growth
Hormone
Also called
somtotropin and is produced by the anterior pituitary. The action of GH is
antagonistic to that of insulin in that it inhibits glucose uptake by the
tissues and stimulates liver glycogenlysis, thus raising the blood glucose
concentration.
Cortisol
and 11–deoxysteriods
Secreted by
adrenal cortex and raises blood glucose concentration primarily by stimulating
glyconeogenesis. They also have some metabolic effects that are antagonistic to
insulin and are sometimes referred to as diabetogenic hormone.
Epinephrine
Also known
as adrenaline, is a catecholamine secreted by adrenal medulla. It increases the
blood glucose level by stimulating glycogenolysis and serves as a back up for
glucagon. It is triggered by physical or emotional stress. This causes
immediate increase in the production of glucose for energy along with an
increase in heart rate, blood pressure and other physiologic effect.
Thyroxine
(T4)
A
tetraiodinated amino acid secreted by the thyroid gland. It promotes
glycogenolysis and can lead to a depletion of glycogen store in the liver. It
also accelerates glucose absorption from the intestine and may lead to a mildly
abnormal, diabetic type of glucose intolerance in hyperthyroid individuals even
though their fasting blood glucose level is usually normal. Although the action
of thyroxine is hyperglycemic, it has an insignificant role in regulating the
blood glucose concentration.
Somatomedins
These are
peptides in the liver in response to stimulation of growth hormone.
Somatomedins are a group of hormones, including somatomedin A, somatomedin C
and insulin like growth factors I and II, that directly promote growth. In
addition to their growth–promoting effects, somatomedins show insulin–like
activity in some tissues, such as adipose tissue. It has been shown that
insulin – like growth factor I and II has a structure similar to that of
insulin.
****** Methods of glucose determination ******
1.
Chemical Methods
a.
Alkaine Copper Reduction Methods
In hot alkaline solution, cupric ions are
reduced to cuprous ions by glucose with the formation of cuprous oxide. Other
reducing sugars are lactose, fructose and pentose. Sucrose is a non–reducing
sugar.
(1) Folin–Wu
The tungstic acid filtrate of whole blood /
plasma / serum is heated with alkaline copper solution. The glucose in the
solution reduces cupric ions and is made to react with phosphomolybdic acid to
form a blue complex of molybdenum blue which is measured colorimetrically and
compared with a standard.
This method lacks specificity since it also
measures saccharoids like ergothione, glutathione, ascorbic acid, uric acid and
creatinine.
A constricted tube is used in this test to
minimize surface tension and thus prevent the reoxidation of cuprous ions by
oxygen. Eighteen percent sodium sulfate may also be added to decrease the
solubility of oxygen and thus achieve the same effect as the constricted tube.
Normal values: 80 – 120 mg/dl
(2) Nelson–Somogyi
This is the most accurate redox method and
believes to be a measure of true glucose, because of the saccharoid free PFF.
The barium sulfate formed acts as an adsorbent to which saccharoids adhere. In
this method, the PFF is made to react with an arsenomolybdate reagent forming a
blue end product of arsenomolybdate blue.
Normal values: 65 – 100 mg / dl
(3) Neocuproine
PFF is made to react with dimethyl
phenanthroline hydrochloride or neocupreine forming a yellow to yellow–orange
cuprous–neocupreine complex.
(4) Benedict’s Method
Same as Folin–Wu except that sodium sulfite
is added which increases the sensitivity of glucose at the expense of
saccharoids and uses a copper reagent of Benedict. The copper reagent is a
modification of the Fehling in which the alkalinity is diminished by using
sodium carbonate instead of the hydroxide and the addition of alanine. The
amino acid forms a cupric salt complex which is unaffected by the non–glucose
substances of the blood.
(5) Shaeffer–Hartman–Somogyi
(Iodometric method)
Cuprous ions formed react with iodine in
acidic solution and excess iodine in the blank and sample is titrated with
thiosulfate. The difference is equal to the reducing sugar present in the
sample.
Advantages:
(a)
A
colorimeter is not required.
(b) It is considered to be the most
accurate for the determination of glucose.
Disadvantage:
(a)
It
is time consuming.
b.
Alkaline Ferric Reduction Methods
(1) Hagedorn–Jensen (Autoanalyzer
method)
In a hot alkaline solution, yellow ferricyanide
ion oxidize glucose to a colorless ferrocyanide ion. Also known as inverse
colorimetry.
Dialysis separates the glucose from red blood
cells and proteins. The dialyzed glucose decolorizes the potassium ferricyanide
to ferrous from and the disappearance of color which is proportional to the
amount of glucose of color which is proportional to the amount of glucose is
measured photometrically.
c.
Condensation Methods
(1) Ortho–toluidine method (Dubowski)
o–toluidine condenses with the aldehyde group
of glucose in a hot acetic acid solution to form an equilibrium mixture of a
glycosylamine and the corresponding Schiff base forming a green chromogen
measurable at 630nm.
This is the most specific non–enzymatic method
for glucose determination. The hot acidic solution is for enolization or enol
formation. However, its use presents a health hazard because o–toluidine is now
classified as carcinogen.
Sources of error:
(a)
Bilirubin
gives falsely elevated values since it may be partially converted to green
pigment biliverdin
(b) Turbidity in the final solution
owing to presence of lipemia or the plasma expander dextran in the specimen
likewise causes falsely high results.
(c)
Sodium
fluoride and EDTA also contribute to the final color of the reaction
(d) Somewhat higher values are
obtained in patients with uremia and galactosemia.
(2) Phenol method
2.
Enzymatic Method
a.
Glucose oxidase method
(1) Saifer–Gerstenfield method
Glucose is measured by the reaction with
glucose oxidase in which gluconic acid and hydrogen peroxide are formed.
Hydrogen peroxide then reacts with an oxygen receptor such as ortho–toluidine
or ortho–dianisidine in a reation catalyzed by peroxidase to form a blue color.
(2) Trinder method
Utilizes a dye, 4–aminophenazone oxidatively
coupled with phenol but has the same principle as Saifer–Gerstenfield.
(3) Gochman method
Utilizes a dye, 3–methyl–2–benzolinome
hydrazone (MBTH) oxidatively coupled with N,N–dimethylaniline (DMA) but has the
same principle as Saifer–Gerstenfield.
(4) Polarographic method
The amount of oxygen consumed in the
oxidation of glucose to gluconic acid is measured using a polarographic oxygen
electrode. This method is precise, linear and free from interferences. Whole
blood should not be used since viable cells use oxygen.
(5) Dextostix
These are reagent strips made of firm
cellulose strips impregnated with highly purified glucose oxidase and chromogen
indicator systems under a semi–permeable membrane which permit approximate
quantitation of blood glucose.
b.
Hexokinase method
This is the most specific method for blood
glucose and is the reference method. This system involves two coupled
reactions:
(1) NADP+ is required as
the cofactor when glucose–6–PO4 dehydrogenase is derived from yeast.
(2) NAD+ is used instead
of NADP+ when the source of glucose–6–PO4 dehydrogenase is bacterial
(Leuconostoc mesenteroides)
(3) Hemolysis interferes with
hexokinase system because red blood cell glucose–6–PO4 dehydrogenase
and 6–phosphogluconate dehydrogenase use NADP+ as substrate.
(4) The hexokinase system can also be
coupled to an indicator reaction using phenazine methosulfate (PMS) and an
iodonitrotetrazolium (INT) so that absorbance can be measured in the visible
range.
c.
Sunderman catalase method
Makes use of catalase, the evolved H2O2 in
the presence of catalase oxidizes methanol to formaldehyde with the latter
measured by a chromatographic acid giving a blue violet color complex.
****** Tests for Diabetes Mellitus ******
1.
Glucose Tolerance Test –
a multiple blood and urine sugar test that rules out diabetes. This is to
detect the presence of diabetes in a patient who is suspected to be diabetic
but whose ordinary routine FBS determination is not or is slightly elevated.
Patients with mild or diet–controlled
diabetes may have a fasting blood glucose levels within normal range but unable
to produce sufficient insulin for prompt metabolism of ingested carbohydrates.
As a result, blood glucose roses to abnormally high levels and the return to
normal is delayed. In other words, the patient has decreased tolerance for
glucose. Therefore, glucose tolerance test are most helpful in establishing a
diagnosis of mild case of diabetes.
Principle: A
normal individual when given a glucose challenge is capable of converting
the
same to glycogen and his glucose level is therefore back to normal in 3
hours.
A diabetic person (because of insulin deficiency) will remove glucose
from
the breakdown at a rate slower than that of a normal individual.
a.
Oral Glucose Tolerance Test
(OGTT)
(1) Janney Isaacson Method or Single
Oral Dose Method
(2) Exton–Rose Method or Divided Oral
Dose Method
Procedure for OGTT:
(1) The patient should have unlimited
physical activity and an unrestricted diet containing at least 150 grams of
carbohydrates for 3 days before the test is performed.
(2) The test should be performed in
the morning after the patient has fasted for 10 – 16 hours.
(3) A fasting blood sample and urine
specimen is obtained. This will serve as baseline.
(4) A solution containing 0.5 grams
of glucose per pound body weight is given to children and a solution containing
100 grams of glucose is given to adults. One hundred grams of glucose is
dissolved in about 200 ml of water and flavored with lemon juice. Commercially
prepared orange juices are now available for this kind of procedure.
(5) Blood and urine specimens are
collected at 30 minutes, 1 hour, 2 hours and 3 hours for analysis for glucose. Normally,
all these urine specimen show a negative reaction. The level of the plasma
glucose at which glucose appears in the urine is called the renal threshold and
is approximately 130 mg / dl.
b.
Intravenous Glucose Tolerance
Test
Since glucose absorption after the oral
ingestion take place through the small intestine, it follows that, when gastric
or intestinal disease is present, the rate of absorption will be affected. This
is the basis for the intravenous method. The same precautions and procedures as
the oral method are followed except for the administration of glucose which is
introduced into the vein.
Normal response to Glucose
Tolerance Test
Blood
Sugar Urine
(mg/dl)
Fasting 80 0
After 30 minutes 155 0
After one hour 145 0
After two hours 75 0
After 3 hours 80 0
In normal persons:
a.
The
fasting blood specimen is normal.
b.
The
30 minute blood specimen does not exceed the fasting blood specimen by more
than 75 mg.
c.
The
one hour blood specimen does not exceed the 30 minutes blood specimen by 10 mg.
·
In
diabetes, the one hour blood specimen exceeds the two hours specimen by 10 mg
or more and the urine specimens are usually positive for glucose.
2.
Post–prandial Blood Glucose Test
It is based on the principle that the glucose
concentration in blood specimens drawn 2 hours after a meal is rarely elevated
in normal individuals, while it is significantly increased in diabetic
patients.
3.
Insulin Tolerance Test
Insulin administered to a normal person in
the post–absorptive state causes a prompt decrease of blood sugar and then a
gradual return to the original level. Normally, the blood sugar level falls to
about 50% of the fasting level in 30 minutes and return to the original level
or above in 2 hours.
4.
Epinephrine Tolerance Test
Epinephrine accelerates glycogenolysis and
promptly increases the blood sugar. This increase of blood sugar following
administration of epinephrine is an index of the quantity and availability of
liver glycogen for maintaining normal blood sugar. Ten minims of 1:1000
solution of epinephrine hydrochloride is injected intramuscularly after a
fasting blood specimen has been taken.
5.
Tolbutamide Diagnostic Test
This test is based on the difference in
response to normal subjects and diabetic individuals to intravenous administration
of a test dose to tolbutamide. Tolbutamide is a compound that stimulates the
pancreas to produce insulin. This test differentiates insulinomas from
hyperinsulinemia states.
Twenty ml of orinase solution (Tolbutamide)
at a constant rate over a 2–3 minute period is injected intravenously after a
fasting blood specimen has been taken. The blood specimen are taken at exactly
20 and 30 minutes later, timing from the mid–point of the injection and the
blood sugar is taken after feeding a high carbohydrate breakfast.
Interpretation:
If the blood glucose value of the 20 minute
specimen is 90% or more of the fasting level, the patient is definitely
diabetic. If the 20 minutes specimen is within the range of 85–89% of the
fasting level, diabetes is probable; the range of 75–84% represents borderline
cases and less than 75% represents normal. The 30 minutes specimen is of value
in confirming the diagnosis.
CLINICAL SIGNIFICANCE OF CARBOHYDRATE DETERMINATION
I. Hypoglycemia
Hypoglycemia
is a syndrome characterized by low plasma glucose levels, usually less than 50
mg/dl (2.8 mmol/L), although not all investigator agree on the exact cutoff
values.
Classification of hypoglycemia
A. Reactive hypoglycemia – occurs because of some stimulus and is
caused by:
1. Factitious hypoglycemia – excessive administration of insulin or
other hypoglycemic agents or by a reduction in gluconeogenesis as a result of
ethanol ingestion.
2. Postprandial hypoglycemia – occur several hours after a meal in
individuals who have had gastrointestinal surgery or have mild diabetes. Relief
is obtained by food intake.
B. Fasting or Spontaneous
hypoglycemia – caused by excessive insulin
secreted by
insulin –producing
pancreatic islet cell tumors (insulinomas), non–pancreatic tumors that produce
substances with insulin–like activity, hepatic dysfunction, glucocorticoid
deficiency, sepsis or depleted glycogen stores.
Symptoms of hypoglycemia
A.
Rapid fall of plasma glucose
(adrenergic symptoms)
1. Sweating
2. Weakness
3. Shakiness
4. Trembling
5. Nausea
6. Hunger
7. Rapid pulse
8. Light–headedness
9. Epigastric discomfort
B.
Gradual fall of plasma glucose to
less than 20 or 30 mg/dl (neuroglycopenia)
1. Headache
2. Confusion
3. Lethargy
4. Seizure
5. Unconsciousness
6. Irreversible brain damage
Causes of hypoglycemia in neonates and children
A. Eclampsia
B. Prematurity
C. Polycythemia
D. Respiratory distress syndrome
E. Gluconeogenic enzyme or counter
regulatory hormone deficiencies
F. Galactosemia
G. Hereditary fructose Intolerance
II. Hyperglycemia
Diabetes mellitus
is the most important disease associated with hyperglycemia. It is
characterized by a deficiency of insulin secretion or action.
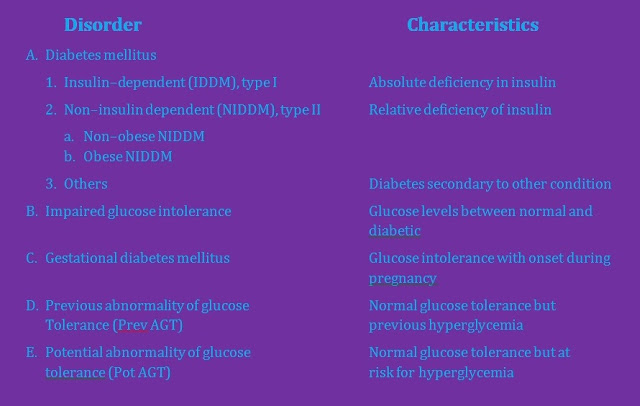
Classification of Diabetes mellitus &
other glucose intolerance:
Primary Diabetes mellitus – based on the activity and state
of the pancreas involving the insulin–producing cells.
A. Insulin Dependent DM (IDDM) or
Type I DM:
1. Occurs at an early age (juvenile
onset).
2. Seen in thin patients.
3. Abrupt onset of symptoms.
4. Absolute deficiency of insulin.
5. Congenital – not hereditary
6. Ketosis prone.
7. Requires insulin treatment.
B. Non–insulin dependent (NIDDM) or Type II DM:
1. Occurs after age 40 (maturity
onset)
2. Seen in obese patients
3. Gradual onset of symptoms
4. A degree of basal insulin
production persists.
5. Hereditary – genetically linked.
6. Ketosis resistant
7. Requires drugs that potentiate
insulin release like oral hypoglycemic agents.
Signs and symptoms of Diabetes
mellitus: The classical triad
1. Polyuria – excessive urination
Ketosis –
overproduction of ketone bodies and result in appearance either in urine
(ketonuria) or blood (ketonemia). Since acetone is volatile, it may be present
in the breath of diabetics, giving it a characteristic sweet “organic” odor.
2. Polydipsia – intake of large
volume of water.
3. Polyphagia – excessive desire to
eat
Post–treatment complications of Diabetes mellitus:
1. Retinopathy leading to blindness,
kidney failure, neurologic defects and microvascular and macrovascular disease.
2. Heart attacks and strokes due
vascular complications
3. Coronary artery disease
4. Gangrene due to diminished blood
flow to the legs and feet because of arteriosclerosis causing lower limb
amputations along with loss of response to normal pressure, minor trauma, susceptibility
to infections, etc.
Secondary Diabetes mellitus – associated with
endocrinopathies.
A. Cushing’s syndrome – a disease of
the adrenal cortex causing excessive secretion of diabetogenic glucocorticoid.
B. Pheochromocytoma – a tumor
involving the chromocytes of the adrenal medulla causing excessive production
of epinephrine and norepinephrine.
C. Acromegaly – a disease
characterized by enlargement of the bones of the hands, feet and skull due to
excessive amount of growth hormone.
Impaired Glucose Tolerance (IGT) – is characterized
by glucose levels that are not normal yet sufficiently abnormal to be
classified in the category of diabetes mellitus. The glucose levels of these
patients may revert to normal or remain borderline, but those patients do have
a greater risk for the development of diabetes mellitus.
Gestational diabetes mellitus (GDM) – is characterized by the onset of or IGT
during pregnancy. After delivery, the patient’s glucose level may revert to
normal or the patient may gent diabetes mellitus later in life.
Previous abnormality of glucose tolerance (PrevAGT) –
is used to refer to individuals who now have a normal glucose levels but who
have previously had abnormal glucose tolerance.
e.g. Women
who have GDM
Obese
individuals with NIDDM
Potential abnormality of glucose tolerance (PotAGT) –
pertains to persons who have normal glucose levels but who are at increased
risk for the development of diabetes mellitus.
e.g. Identical
twins
Sibling
or offspring of diabetic patient
III. Inborn errors of carbohydrate
metabolism
A.
Glycogen storage disease
B. Disorder of
fructose metabolism
1. Hereditary fructose intolerance –
caused by a deficiency of fructose–1–phosphase aldolase, thus causing the
accumulation of fructose–1–phosphate in cells. The ingestion of fruit or
sucrose produces vomiting, hypoglycemia, hepatomegaly and failure to thrive.
2. Fructose–1,6 diphosphate
deficiency – results from lack of fructose 1,6– diphosphatase, an enzyme
necessary in the formation of glucose from pyruvate. Infants with this rare
disease have fasting hypoglycemia, lactic acidosis, hepatomegaly and a poor
prognosis.
3. Essential fructosuria – benign condition
caused by a deficiency of hepatic fructokinase. It is characterized by high
fructose levels in serum and urine after ingestion of sucrose or fructose.
C.
Mucopolysaccharide storage disease
Mucopolysaccharides
are structural components of cartilage, bone, skin and other connective
tissues. They consist of repeating disaccharide units that contain a hexosamine
(usually acetylated), a uronic acid and often a sulfate group attached to the
hexosamine. The three classes of mucopolysaccharides are dermatan sulfate, heparan
sulfate and keratan sulfate.
Mucopolysaccharides
are hereditary disorders caused by a deficiency of one or more lysosomal
enzymes. The mucopolysaccharides (glucosaminoglycans) accumulate in various
tissues and are excreted in the urine.
Hurler’s
syndrome is the prototype of all mucopolysaccharide storage diseases. It is a
severe, progressive disorder characterized by corneal clouding and death
usually before the age of 10. Individuals with this disorder have coarse faces,
skeletal abnormalities, developmental delay and hepatosplenomegaly.
IV. Glycated hemoglobin
Glycated hemoglobin
provides an index of the mean concentration of blood glucose over the preceding
two months. The test is useful in determining compliance with therapy and the
extent to which satisfactory diabetic control has been achieved.
Formation of glycated hemoglobin
HbA contains
several minor hemoglobin components, identified as HbA1a, HbA1b
and HbA1c. These are modifications of HbA and are collectively
referred to as glycated hemoglobin, glycosylated hemoglobin, “fast hemoglobin”
or glycohemoglobin.
HbA1c
is the most defined by the hemoglobin fraction and is formed by a non–enzymatic
reaction, referred to as glycation, between glucose and the N–terminal valine
amino acid of each beta chain of HbA to form a labile Schiff’s base (pre–A1c)
with an aldininie structure. As the red cell circulates, some of the aldiminine
undergoes a slow, irreversible Amadoric arrangement to yield a stable ketoamine
(HbA1c). This reaction is continuous
over the 120–day life span of the RBC and is proportional to the concentration
of glucose in blood.
1. Methods based on charge
differences
a. Ion exchange chromatography
b. High performance liquid
chromatography
c. Electrophoresis
d. Isoelectric focusing
a. Hydroxymethylfurfural (HMF) /
thiobarbituric acid colorimetric method
3. Method based on structural
differences
a. Affinity chromatography
Normal
values: HbA1 5 – 8%
HbA1c 3 – 6%







No comments:
Post a Comment