Classification of Gram–Positive Bacilli:
A. Spore–Forming Group
1. Aerobic
or Facultative Anaerobic
a. Bacillus Group
2. Obligate
Anaerobes
a. Clostridium Group
B. Non–Spore Forming Group
a.
Listeria Group
b. Erysiphelothrix Group
2.
Irregular shape
a.
Acid Fast
(1) Mycobacterium Group
a.
Non–Acid Fast
(1)
Corynebacterium
Group
(2) Propionibacterium Group
b.
Filamentous
cells
(1)
Actinomyces
Group
(2) Nocardia Group
THE BACILLUS GROUP
General characteristics:
1. Most members are saprophytic organisms prevalent in soil, water, and air and on vegetation, some are insect pathogens. They may occasionally produce disease in immunocompromised humans.
2. Typical cells have square end and are arranged in long chains resembling “jointed bamboo fishing rods.”
3. Most reliable diagnostic characteristics of this genus is the centrally located spores.
4. They are encapsulated during growth in an infected animal, but capsules cannot be demonstrated unless culture in medium containing sodium bicarbonate with 5 – 10% CO2 at 37oC.
5. They are motile due to peritrichous flagella except Bacillus anthracis.
6. Variations occur with respect to virulence, spore formation and colony form. Virulence is associated with rough colonies.
7. Cell walls contain meso–diaminopimelic acid and several species produce a carbohydrate capsule. Due to their thick cell wall coat, spores may not be observed in gram staining.
8. InhA1 (Immune Inhibitor A) is a particularly well–studied protease that has homologues in all three species, Bacillus anthracis, Bacillus cereus, and Bacillus thuringiensis. InhA1 disturbs the host coagulation machinery by cleaving von Willebrand factor and its regulator ADAMTS13. The enzyme also cleaves zonula occluden–1 resulting in disruption of the blood–brain barrier. InhA1 is required for efficient escape from macrophages.
9. It
is resistant to heat and drying and some chemical disinfectants.
Classification of Bacillus:
1. Group I – Gram positive, produces central of terminal, ellipsoidal or cylindrical spores that do not distend the sporangium.
a. Subgroup 1 – has large cell (more than 0.9 μm)
(1) Bacillus anthracis, Bacillus cereus, Bacillus mycoides, Bacillus megaterium & Bacillus thuringiensis
b. Subgroup 2 – has small cells (less than 0.9 μm) and has simple nutritional requirements.
(1) Bacillus subtilis, Bacillus coagulans, Bacillus licheniformis and Bacillus pumilus
2. Group II – Gram variable with central or ellipsoidal spores and swollen sporangia with complex nutritional requirement: Bacillus circulans and Bacillus coagulans, Bacillus stearothermophilus, Bacillus polymyxia, Bacillus brevis, Bacillus macerans.
3. Group III – Gram variable, sporangia swollen with terminal or subterminal spores and has the most fastidious and complex nutrition: Bacillus sphaericus, Bacillus pasteurii, Bacillus pantothenticus.
Cultural characteristics in general:
1. Bacillus sp. may grow well on all commonly used Blood Agar but sheep blood agar is recommended for isolation of Bacillus organisms from sterile sites. Phenyl ethyl alcohol (PEA) agar is recommended for contained sites.
2. Bicarbonate agar may induce capsule formation and all media should be incubated at 35oC in air or in CO2 incubator.
3. On 5% sheep blood agar, colonies of Bacillus cereus are alpha or beta hemolytic with lavender color, small, shiny, compact, and feathery while colonies of other Bacillus species are large, flat, and dull with ground glass appearance.
4. Some species undergo pigmented formation which is increased by carbohydrate in media.
5. There are times Bacillus species resemble gram negative rods especially the non–fermenting gram–negative bacilli. To differentiate them, note the following factors:
a. Bacillus
species do not grow on enteric agars.
b. Bacillus
species are sensitive to vancomycin whereas gram negative non– fermenting organisms
are not.
c. KOH test, gram negative rods show a viscous thread.
Classification of Bacillus infection:
1. Local – involves the eye (keratitis, conjunctivitis, orbital abscess)
2. Mixed – infection in the company of other organisms (wound infection, burns, pneumonia).
3. Disseminated – organisms cultured from blood and CSF of a seriously ill person (bacteremia, endocarditis, meningitis)
BACILLUS ANTHRACIS
Cultural characteristics:
1. They are spore-forming, and causes anthrax disease, which is primarily a zoonotic disease found all over the world.
2. On BAP, it is non–hemolytic, large, rough, and opaque with comma shaped outgrowths, Medusa head colony or comet tail colony. Colonies are also described as cut–glass appearance.
3. On gelatin stabs, liquefaction takes place, and it exhibits inverted fir tree.
4. The poly-γ-D-glutamic acid (PGA) capsule provides protection of the bacterium from phagocytosis and is more commonly made of polysaccharide.
5. Anthrax is a category A biological weapon; as such, it is an ideal candidate for biological terrorism.
6. It has three different strains, two of which are encapsulated and virulent.
Toxins produced by Bacillus anthracis:
1. Edema Factor (EF) – also known as Calmodulin–dependent adenylate cyclase. It converts adenosine triphosphate to cyclic adenosine monophosphate, thereby increasing intracellular cyclic adenosine monophosphate levels leading to the edema characteristic of the disease.
2. Lethal Toxin (LeTx) – a major virulence factor secreted of the bacteria. It is composed of two proteins, PA (protective antigen) and LF (lethal factor). PA transports the LF inside the cell, where LF, a zinc–dependent metalloprotease cleaves the mitogen activated protein kinase kinase (MAPKK) enzymes of the mitogen activated protein kinase (MAPK) signaling pathway, thereby impairing their function. This disruption of the MAPK pathway, which serves essential functions such as proliferation, survival and inflammation in all cell types, results in multisystem dysfunction in the host. The net result is an impaired innate and adaptive immune response.
3. AnthrolysinO (ALO) – is a cholesterol–dependent cytolysin with properties similar to pore-forming toxins of other Gram–positive pathogens. Initially discovered as a hemolysin, ALO has lytic activity against phagocytes, decreases the barrier function of human polarized epithelial cells, and appears to play a role in gut epithelial disruption during infection.
Strains of Bacillus Anthracis:
1. Ames strain – first isolated from diseased 14–month old beefmaster heifer that died in Sarita, Texas in 1981. It is now widely used as the reference strain. The Ames lineage strains were isolated from Inner Mongolia in early 1955 and primarily distributed in the northern regions of China. It has also been found in Korea, Kazakhstan, and Denmark. It was also the same strain that was used as a bioterrorism agent in the 2001 US Anthrax Letter Attack.
2. Vollum strain – closely related to CDC 684 strain. Oftentimes designated as A0488 strain. Contains both the pX01 and pX02 megaplasmid, non–hemolytic, sensitive to gamma phage and produces the protective antigen and the poly–D–glutamic acid capsule.
3. Sterne strain – the avirulent strain which has no ability to cause illness. It doesn’t produce a capsule, which protects it from being consumed and destroyed by our defensive immune system cells. It has been used, however, in the production of vaccine for anthrax.
There is now clear evidence that some of the virulence factors of Anthrax are mediated by plasmids. The two megaplasmids associated with virulence is the pX01 and pX02.
1. Plasmid pXO2 (60 MDa) carries the genes required for synthesis of an antiphagocytic poly-d-glutamic acid capsule facilitating host immune system evasion.
2. Plasmid pXO1 (110-MDa) is required for synthesis of the three anthrax toxin proteins, edema factor (EF), lethal factor (LF), and protective antigen (PA).
3. Manganese ion–binding protein (MntA) – recently identified as a major virulence element. The Bacillus anthracis MntA mutant was as attenuated as a pXO2 strain, despite its ability to express all the known toxins and the capsule.
Clinical types of anthrax:
1. Cutaneous anthrax or Malignant Pustules or Charbon (94% of cases) – obtained by skin contact from infected animals. The incubation period has been noted between 1 and 19 days, usually 2–7 days.
2. Pulmonary anthrax or Woolsorter’s disease (5% of cases) – inhalation of spores while handling wool. The incubation period is usually 1 to 7 days.
3. Intestinal anthrax or violent enteritis (1% of cases) – obtained by the ingestion of improperly cooked infected meat. The incubation period is usually 1 to 7 days.
4. Injection anthrax – acquired by those injecting heroin.
Laboratory diagnosis:
1. Stained smears: M’fadyean’s reaction
M'Fadyean uses 1% methylene blue to stain smears of blood from dead farm animals. The darkly stained anthrax bacilli were within capsules that were colored pink rather than blue.
2. PLET medium (Polymyxin–Lysozyme–EDTA–Thallous acetate) – produces a tenacious type of colony like a beaten egg when touched with a loop.
3. Gamma Phage Lysis Assay – is a common diagnostic method for identification of Bacillus anthracis and is based on the bacterium’s susceptibility to lysis. This test has been shown to be 97% specific.
4. String of Pearl reaction – separates virulent from avirulent form of Bacillus anthracis. This can be demonstrated following 5 – 6 hours incubation of Bacillus anthracis on a medium containing 0.05 to 0.5 units of penicillin G. The cells become large and spherical and occur in chains resembling string of pearl. The test was used historically and currently is not recommended.
5. Ascoli test – is a thermoprecipitation test to detect whether tissues or animal hides have been contaminated with spores of B. anthracis.
Ascoli test is done by first boiling the macerated small piece of tissue in saline solution with acetic acid for 5 minutes. After cooling, the filtrate is layered over immune serum and then observed for the appearance of a ring of precipitate at the interface.
6. Lectin
agglutination assays – Bacillus anthracis was agglutinated
by several lectins, including those from Griffonia simplicifolia, Glycine max,
Abrus precatorius, and Ricinus communis. Some strains of Bacillus cereus var.
mycoides were strongly reactive with the lectin from Helix pomatia and weakly
reactive with the Glycine max lectin. The differential interactions between
Bacillus species and lectins afforded a means of distinguishing Bacillus
anthracis from other bacilli.
BACILLUS CEREUS
Characteristics:
1. They are catalase–positive, endospore–forming, motile bacterium.
2. The endospores are resistant to heat, radiation, disinfectants, and desiccation, and their adhesive character facilitates their attachment to processing equipment and resistance to cleaning procedures.
3. It was first isolated from air in a cow–shed and was characterized as a highly motile bacterium, generally appearing as single cells but occasionally forming longer threads and causing rapid liquefaction of gelatin. In the second half of the twentieth century, it was recognized as a common food contaminant.
4. It is also responsible for a variety of severe extra–gastrointestinal infections. Often neonates, elderly or immunosuppressed patients are affected, but infections of immunocompetent persons are also known.
5. Certain strains of Bacillus cereus have been reported to promote plant growth and suppress plant diseases.
6. On PEMBA (Polymyxin–Pyruvate–Egg Yolk–Mannitol–Bromthymol blue) Agar, the colonies of Bacillus cereus are rough, dry and crenated or rhizoid with distinct bright blue color.
Two types of food poisoning produced by Bacillus cereus:
1. Emetic type – it has short incubation period (1 – 5 hours), the emetic toxin (cereulide) produced by the organism while still in food implicated is ingested such as fried rice. Usually, the emetic form of disease is self–limiting and symptoms disappear after 6–24 hours.
2. Diarrheal type – it has long incubation period (10 – 12 hours), bacteria is ingested and produces toxin (enterotoxin) in the digestive tract. Vegetable soup, uncooked meat, poultry, and desserts are the foods involved.
Toxins produced by Bacillus cereus:
Two protein toxin complexes (Nhe and Hbl) and a single protein toxin (CytK) have been described to contribute to the diarrheal type of food poisoning while the cereulide toxin have been describe for the emetic type.
1. Cereulide – the causative agent of the emetic type of food poisoning. Cereulide is a depsipetide toxin, composed of alternating α-amino and α-hydroxy acids (D-O-Leu–D-Ala–L-O-Val–L–Val), that is structurally related to the potassium ionophore valinomycin. Cereulide is usually preformed in food and the onset of symptoms occurs between 15 minutes and 6 hours after uptake of contaminated food.
2. Non–hemolytic Enterotoxin (Nhe) – a three component “non–hemolytic” enterotoxin composed of NheA, NheB and NheC component. When Nhe was first discovered in the 1990s, no hemolysis on sheep blood agar could be detected, thus it was mistakenly described as “non-hemolytic.” However, Nhe shows hemolytic activity against erythrocytes depending on the species used.
3. Hemolysin BL (Hbl) – a three component “hemolytic” enterotoxin composed of L1, L2 and B component which was thought to contribute to diarrheal food poisoning and necrotizing infections such as endophthalmitis. The HBL components are nontoxic, but when combined they exhibit a variety of toxic activities including hemolysis, cytotoxicity, vascular permeability, dermonecrosis, enterotoxicity, and ocular toxicity.
4. Cytotoxin K (CytK) – a single component toxin and like any other beta–barrel pore-forming toxins, spontaneously forms oligomers which are resistant to sodium dodecyl sulphate (SDS), but not to boiling. CytK represents a pore-forming toxin linked with human cases of necrotic enteritis.
BACILLUS THURINGIENSIS
It was first isolated in 1901 in Japan from infected silkworm larvae (Bombyx mori). The bacterium was identified as the causal agent of the Sotto Disease (sudden-collapse disease), a lethal infection of this economically important insect. Subsequently, Germans isolated the bacterium from the cadaver of flour moth larvae (Ephestia kuehniella) collected in a mill in Thuringia, and named it Bacillus thuringiensis, after the German province. Bacillus thuringiensis was rapidly commercialized and used as a biopesticide for pest control.
Insecticidal proteins secreted by Bacillus thuringiensis:
1. Sip (secreted insecticidal protein) – Sip1A is active against coleopteran insects and was isolated from a Bacillus thuringiensis strain harboring a coleopteran-specific cry3 gene.
2. Vip (vegetative insecticidal proteins) – the toxin Vip3A is specifically toxic against lepidopteran insects and is generally produced by strains also producing Cry1A lepidopteran–active toxins.
The insecticidal activity of the exported proteins Vip and Sip and their association with Cry proteins showing related activity spectrum suggest that the larvicidal activity of the Cry proteins is reinforced by the secretion of Sip and Vip proteins, thus increasing the entomopathogenicity of the Bacillus thuringiensis cells multiplying in the infected insect larvae.
The following tests are suggested for species identification of Bacillus group of organisms:
1. Catalase
production
2. Voges–Proskauer
reaction
3. Growth
in anaerobic jar
4. Growth
at 50oC
5. Growth
at 65oC
6. Growth
in 7% NaCl
7. Production
of acid and gas from glucose
8. Reduction
of nitrate to nitrite
9. Hydrolysis
of starch
10. Casein
decomposition
11. pH in Voges–Proskauer broth
API systems supplemented with spore examination, motility testing and gas from carbohydrate fermentation are the rapid, accurate and reproducible test than the classic biochemical test.
THE GENUS CLOSTRIDIUM
General characteristics:
1. Their natural habitat is the soil or the intestinal tract of animals and humans, where they live as saprophytes. Among the pathogens are those causing botulism, tetanus, and gas gangrene.
2. All species are gram positive rods, and all can produce spores which are subterminally or terminally located.
3. Endospores produced by Clostridium do not stain easily. Endospores are stained by Wirtz–Conklin method where malachite green is used for staining and heat is used to penetrate stain. The rest of the cell is then decolorized and counterstained a light red with carbolfuchsin.
4. The outstanding characteristic of anaerobic organisms is their inability to utilize oxygen as the final hydrogen acceptor. They lack cytochrome oxidase and superoxide dismutase.
5. Toxin production is dependent on metalloproteinases.
6. Capsules are demonstrated in the smears of wounds but not in cultures. All Clostridia are non-encapsulated except Clostridium perfringens.
7. All Clostridia are single–hemolytic except Clostridium perfringens which is double hemolytic.
Classification of Toxins produced by Clostridia:
1. Clostridial β–pore forming toxins (PFTs) – they are secreted as soluble monomers rich in β-strands, which recognize a specific receptor on target cells and assemble in oligomers. Then, they undergo a conformational change leading to the formation of β-barrel, which inserts into the lipid bilayer forming functional pore.
a. Clostridial cholesterol–dependent cytolysins (CDCs) – the pore–forming mechanism of these toxins exhibits two hallmark characteristics: an absolute dependence on the presence of membrane cholesterol and the formation of an extraordinarily large pore which disrupt the plasma membrane integrity.
b. Clostridial heptameric β–PFT – they induce small pores which trigger signaling cascades leading to different cell responses according to the cell types and toxins.
(1) Aerolysin Family – the toxins share a similar monomeric architecture, with a variable membrane–binding (MB) domain and a structurally conserved pore-forming (PF) region, presenting five β-strands with an insertion loop [stem loop (SL)] between strands β2 and β3.
(2) Staphylococcal Alpha Hemolysin Family
2. Large Clostridial Toxins (LCT) – they invade host cells by binding specific cell surface receptors, ultimately leading to internalization into acidified vesicles. Acidic pH promotes conformational changes within LCTs, which culminates in translocation of the N–terminal glycosyltransferase and cysteine protease domain across the endosomal membrane and into the cytosol, leading first to cytopathic effects and later to cytotoxic effects.
a. Clostridial Neurotoxins – they proteolyse key components of neuroexocytosis. Botulinum neurotoxins inhibit neurotransmission at neuromuscular junctions, whereas tetanus toxin targets the inhibitory interneurons of the CNS. Examples are Tetanus neurotoxin (TeNT) and the botulinum neurotoxins (BoNTs).
b. Large Clostridial Glucosylating Toxins (LCGT) – the name was derived based on their glucosyltransferase activity. They enter the cells by receptor–mediated endocytosis and translocate from early endosomes upon an acidification step.
c. Clostridial Binary Toxins – consists of two independent proteins including an enzymatic component and a binding component (BC) which are encoded by distinct, yet adjacent, genes. The N-terminal domain 1 contains the binding sites for enzymatic components, and the C–terminal domain 4 is involved in recognition of the cell surface receptor. Clostridial binary toxins are involved in necrotizing enteritis and diarrhea in animals and occasionally in humans.
Both the Clostridial Binary Toxins and the Large Clostridial Glucosylating Toxins alter the actin cytoskeleton by enzymatically modifying the actin monomers and the regulatory proteins from the Rho family, respectively.
Classification of Clostridia:
1. Neurotoxic Group
a. Clostridium
botulinum (see discussion below)
b. Clostridium tetani (see discussion below)
2. Histolytic Group / Gas Gangrene Group
a. Clostridium chauvoei – is of veterinary importance as a causative pathogen of blackleg (Clostridial myositis), a highly fatal gas gangrenous infection of cattle and sheep. Other susceptible hosts reported so far are limited to animals, including goats and swine.
b. Clostridium histolyticum (now Hathewaya histolytica) – is a pathogenic anaerobe that causes gas gangrene. The collagen extracted from the bacteria is used to treat Dupuytren disease (DD), a disease of pathological collagen production and deposition in the hands. Seven collagenases have been isolated from the culture filtrate of the bacteria, namely α, β, γ, δ, ɛ, ξ, and η collagenases. They are all zinc–dependent matrix metalloproteinases whose unique specificity is in the hydrolysis of collagen molecules via the peptide bond between the Gly–Pro and residue X of the tripeptide repeat unit (where residue X is often proline or hydroxyproline).
c. Clostridium septicum – in humans, it causes gas gangrene in normal hosts following deep penetrating or crushing injuries after trauma. It is also recognized as the principal cause of "spontaneous" or "atraumatic" gas gangrene in patients suffering from gastrointestinal maladies, including adenocarcinoma of the colon. The most common predisposing factors for spontaneous infections are hematological and colorectal malignancies. It is known to produce four types of toxins: alpha, beta, gamma, and delta toxin. The best studied of these is α-toxin, a potent pore-forming toxin that is released extracellularly.
d. Clostridium novyi – commonly found in the gut of pig and are responsible for decomposition of the carcass following death.
(1) Clostridium novyi Type A is a bacterium that has recently been associated with a number of fatalities among drug injecting addicts.
(2) Clostridium novyi Type B causes Infectious Necrotic Hepatitis (INH) that typically affects sheep, in which it is also referred to as “black disease” given the dark discoloration of the subcutaneous tissue caused by intense venous congestion seen in the carcasses of animals dying from this disease.
(3) Clostridium novyi Type C is not known to induce illness in and is typically considered nonpathogenic toward laboratory animals.
(4) Clostridium novyi Type D (formerly known as Clostridium haemoliticum) – a well–known animal pathogen and the cause of bacillary hemoglobinuria or red–water disease primarily in cattle but also, more rarely, other animals, such as sheep, horses, buffaloes, hogs, and elks.
e. Clostridium perfringens (see discussion below)
3. Enterotoxic Group
a. Clostridium colinum – a close relative of Clostridium piliforme, causes ulcerative enteritis in young game birds, chickens, turkeys, and occasionally other avian species.
b. Clostridium piliforme – causes Tyzzer Disease (TD), a form of bacterial enteritis. Infection with this organism is more commonly described in small rodents such as mice and hamsters, although it has also been described in guinea pigs as well as other mammalian groups.
c. Clostridium septicum (see discussion above)
d. Clostridium difficile (see discussion below)
e. Clostridium sordellii (see discussion below)
f. Clostridium perfringens (see discussion below)
CLOSTRIDIUM PERFRINGENS OR CLOSTRIDIUM WELCHII
Characteristics:
1. They are strictly anaerobic bacteria that can migrate across surfaces using a type of gliding motility that involves the formation of filaments of bacteria lined up in an end–to–end conformation.
2. It produces spores that can persist in the environment and can resist heat.
3. It causes stormy fermentation of milk due to excessive production of gas.
Toxins produced by Clostridium perfringens:
Clostridium perfringens causes numerous gastrointestinal infections in most mammalian species. These infections are generically called enterotoxemia because toxins produced in the intestine may be absorbed into the general circulation. This microorganism can produce up to 16 toxins in various combinations, including lethal toxins such as perfringolysin O (PFO), enterotoxin (CPE), and beta2 toxin (CPB2).
|
Toxinotype |
CPA |
CPB |
ETX |
ITX |
CPE |
CPB2 |
|
A |
+ |
– |
– |
– |
+/– |
+/– |
|
B |
+ |
+ |
+ |
– |
+/– |
+/– |
|
C |
+ |
+ |
– |
– |
+/– |
+/– |
|
D |
+ |
– |
+ |
– |
+/– |
+/– |
|
E |
+ |
– |
– |
+ |
+/– |
+/– |
1. Alpha toxin (CPA) – CPA in intestinal disease of mammals is controversial and poorly documented, but there is no doubt that this toxin is essential in the production of gas gangrene of humans and several animal species.
This toxin is a zinc–dependent phospholipase C which degrades phosphatidylcholine and sphingomyelin, both components of the eukaryotic cell membranes, causing damage to the membrane of erythrocytes and other cells from many animal species.
2. Beta toxin (CPB) – CPB produced by Clostridium perfringens types B and C is responsible for necrotizing enteritis and enterotoxemia mainly in neonatal individuals of several animal species.
3. Beta2–toxin (CPB2) – a necrotizing and lethal toxin. CPB2 toxin could act as a potential pore–forming toxin similar to other enterically–active clostridial toxins. The toxin was shown to be highly susceptible to proteolytic cleavage by trypsin.
4. Epsilon toxin (ETX) – ETX is an example of an aerolysin–like, pore–forming toxin. It is considered the major virulence factor of Clostridium perfringens types B and D. ETX is the third most potent clostridial toxin after botulinum toxin and tetanus toxin, with a mouse lethal dose of 100 ng/kg. ETX is secreted as a prototoxin (32,981 Da), which is converted into a fully active toxin (~1000 times more toxic than the prototoxin) when activated by proteases such as trypsin, chymotrypsin, and a metalloproteinase named lambda toxin that is produced by Clostridium perfringens.
5. Iota toxin (ITX) – ITX is a clostridial binary toxin. These toxins have a common structure consisting of two independent protein components that are not covalently linked, one being the binding component (Ib, 100 kDa), and the other the enzymatic component (Ia, 45 kDa). Both components are required for biological activity.
6. Enterotoxin (CPE) – CPE is a 319 amino acid protein consisting of an N–terminal domain, which is important for pore formation and cytotoxicity, and a C–terminal domain that mediates receptor binding. Although CPE lacks sequence homology with other toxins, its C–terminal receptor-binding domain structurally resembles that of some Bacillus thuringiensis cry toxins.
7. Perfringolysin O (PFO) – also called theta toxin does not seem to play a direct role as the main virulence factor for animal diseases, but it may have a synergistic role with CPA–mediated gangrene and ETX–mediated enterotoxemia. PFO is secreted as a water–soluble monomer that recognizes and binds membranes via cholesterol.
8. TpeL toxin – a recently discovered toxin. TpeL is a trypsin–sensitive toxin and causes diseases originating in the intestines. It is a sporulation–regulated toxin and cytotoxic, causing cell rounding.
Enzymes produced by Clostridium perfringens:
Clostridium perfringens produces three sialidases, NanH, NanI, and NanJ. These enzymes can function by upregulating the production of toxins associated with intestinal infections, enhancing the cytotoxicity of toxins, increasing the adhesion of Clostridium perfringens to host cells, and producing substrates used for bacterial growth and metabolism.
Sialidases, also known as neuraminidases (E.C.3.2.1.18), hydrolyze the α-glycoside bond of the terminal sialic acid in glycoproteins and glycolipids to produce free sialic acid. Sialidases are the key enzymes responsible for the catabolism of oligosaccharides containing sialic acid.
One of the coping strategies bacteria use to respond to limited enteral nutrients is the production of sialidases, which enable sialic acid to be obtained from the host as a source of carbon, nitrogen, amino acids, and energy.
Two classical inhibitors of Clostridium perfringens sialidases, Siastatin B (SB), and N–acetyl–2,3–dehydro–2–deoxyneuraminic acid (NADNA), have been shown to inhibit Clostridium perfringens sialidase activity. Sialidase inhibitors may be a potential candidate for the development of drugs against human non–foodborne gastrointestinal disease such as antibiotic-associated diarrhea and sporadic diarrhea, and the development of highly effective sialidase inhibitors will be an important direction in generating anti–Clostridium–perfringens infection drugs soon.
Clostridium perfringens infection
1. Enteritis Necroticans or Acute hemorrhagic necrotizing enteritis (AHNE) or Pigbel or Fire belly or Darmbrand – is a potentially fatal infection, triggered by beta toxin produced by Clostridium perfringens type C and characterized by extensive hemorrhagic, inflammatory, or ischemic necrosis that mainly affects the small bowel, clinically presenting as diarrhea, hematochezia, abdominal pain, and hypotensive shock. It develops after consumption of rancid or improperly cooked meat.
2. Gas gangrene or Clostridial myonecrosis – this disease can arise when the anaerobic bacterium is introduced into muscle tissue, often because of a traumatic injury. The disease is characterized by rapid spreading of tissue necrosis within the muscle (thus the term myonecrosis), which can lead to death caused by systemic toxemia and shock, with very high mortality rates, if not promptly treated.
3. Food poisoning – acquired after eating foods that have been sitting out for an hour or two due to the spores from the bacteria. It is commonly found in red meat and poultry but less common in fish and vegetables.
Types of media used for Clostridium perfringens:
|
Purpose |
Media |
Note |
|
Bacteria isolation from food sample |
Tryptose-Sulfite-Cycloserine (TSC) agar |
produces black colonies, 1 to 5 mm due to the production
of ferrous sulfide |
|
Shahidi Ferguson Perfringens (SFP) Agar |
produces black colonies, 2 to 3 mm in diameter,
surrounded by zones of opaque precipitate |
|
|
Rapid Perfringens Medium (RPM) |
A liquid medium with a litmus milk base. Can also detect
gelatin hydrolysis. Stormy fermentation reaction is a positive result. |
|
|
Demonstration of spore enterotoxin |
Duncan and Strong (DS) medium |
The original formula uses starch to ferment sugar |
|
Modified Duncan and Strong medium |
Starch was replaced with raffinose to ferment
sugar |
|
|
Peptone–Bile– Theophylline (PBT) |
Contains bile, bicarbonate and quinoline |
|
|
Peptone–Bile– Theophylline–Starch (PBTS) |
Starch is added to the formulation |
|
|
Tortora medium |
Contains thiotone, starch, dichloridic thiamine, magnesium
sulfate, potassium dihydrogen phosphate, sodium phosphate dibasic
heptahydrate |
Identification test for Clostridium perfringens:
1. Nagler Test – determines the ability of microorganisms to produce lecithinase. Lecithinase producing organisms are identified by a zone of opalescence on Egg Yolk Agar. It uses an antitoxin to the alpha toxin which prevents hydrolysis of phospholipids on the side of the plate being tested. The popularity of the Nagler test has declined because the antitoxin has not been widely available.
2. Reverse CAMP test – alpha toxin producing Clostridium perfringens and group B, β–hemolytic streptococci grow in a characteristic pattern on blood agar. The formation of arrowhead hemolysis is a positive result.
CLOSTRIDIUM TETANI
Characteristics:
1. They have a tennis racket or drumstick appearance because of the terminally located spores. They are strictly anaerobic, motile, spore forming, gram–positive rod that persists in soils, manure, and within the gastrointestinal tract.
2. Unlike botulinum toxin, which remains at the neuromuscular junction to cause a flaccid paralysis, tetanus toxin is transported within the motor nerves to the central nervous system (CNS).
3. On BAP, they are beta–hemolytic.
Toxins produced:
1. Tetanospasmin – is a neurotoxin that inhibits the release of γ-aminobutyric acid (GABA) and results in a variety of clinical signs commonly associated with tetanus including muscle spasms and rigidity, trismus (lockjaw), dysphagia, tendon rupture, opisthotonos, respiratory difficulty, and death.
2. Tetanolysin – is an oxygen–sensitive hemolysin related to streptolysin and the theta–toxin of Clostridium perfringens. It may play a part in establishing infection at the site of inoculation, but it has no other known role in pathogenesis of the disease.
Infections produced by Clostridium tetani:
Tetanus or Lockjaw or Trismus – exhibited muscle stiffness of lower jaw followed by spasms of masseter muscle. Spores germinate in the tissue to vegetative cell to produce toxins. Germination of spores is aided by the following: Necrotic tissue, calcium salts, associated pyogenic infections.
Risus sardonicus – the peculiar smile of tetanus. Gangliosides of brain are responsible for binding the tetanospasmin. To prove this statement, Wassermann–Takaki phenomenon was invented.
Wassermann–Takaki phenomenon is the fixation of tetanus toxin by the grey matter of nervous tissue. In experimental model, when tetanus toxin was mixed with an emulsion of guinea pig brain, its toxicity was reduced or abolished. Injection of brain emulsion gave some protection to mice injected with tetanus toxin 24 hours later.
CLOSTRIDIUM BOTULINUM
Characteristics:
1. They are anaerobic, spore forming bacterium that produces a protein with characteristic neurotoxicity. The spores are sub–terminally located.
2. On BAP, it exhibits alpha–hemolysis.
|
CLOSTRIDIUM
BOTULINUM GROUP BASED ON PHYSIOLOGICAL DIFFERENCES AND 16s rRNA SEQUENCING |
||||
|
|
I |
II |
III |
IV |
|
Toxin Type |
A,B,F |
B,E,F |
C,D |
G |
|
Proteolysis |
+ |
– |
– |
– |
|
Optimal Growth Temperature |
35
– 40oC |
18
– 25oC |
40oC |
37oC |
|
Minimum Growth Temperature |
10oC |
3.3oC |
15oC |
No
data |
|
Spore heat resistance |
112oC |
80oC |
104oC |
104oC |
|
Risk Group |
Humans |
Humans |
Animals
and Birds |
Unknown |
|
Toxin gene location |
Chromosome |
Chromosome |
Phage |
Plasmid |
Seven antigenic varieties of toxins:
Botulinum toxin is a neurotoxin also called as the “miracle poison.” It is one of the most poisonous biological substances known. Botulinum neurotoxins (BoNTs) are zinc–dependent endopeptidases produced by members of the bacteria.
Botulinum toxin was approved for the treatment of numerous disorders of spasticity and a host of other conditions. Currently it is used in almost every subspecialty of medicine. In 2002, the FDA approved the use of Botox (Botulinum toxin A) for the cosmetic purpose of temporarily reducing glabellar forehead frown lines.
1. Serotype Type A – considered as a foodborne pathogenic bacterium detected in canned, packed, preserved, and home-made foods. It secretes the most poisonous potent Clostridium botulinum neurotoxin serotype A (BoNT/A) known to humans and induces a potentially fatal paralytic condition in humans and various animal species.
Serotype A is the only commercially available form of botulinum toxin for clinical use. Two preparations of botulinum toxin A exist: Dysport and Botox
2. Serotype Type B – the toxin produced is used for the treatment of cervical dystonia and sialorrhea.
3. Serotype Type C – produces limberneck (Western Duck Sickness) in fowl. The turkey vulture is the only animal host known to be resistant to the disease.
4. Serotype Type D – produces botulism in cattle.
5. Serotype Type E – found in water and marine wildlife. Has routinely been isolated from fish and seafood products and has caused a number of illness outbreaks associated with seafood consumption. The Type E toxin is also produced by Clostridium butyricum.
6. Serotype Type F – found in home processed venison jerky. This serotype is little studied primarily because they are poor producers of toxin making purification difficult. The Type F toxin is also produced by Clostridium baratii.
7. Serotype Type G – found in soil, autopsy material and amniotic fluid. It produces a toxin complex that is composed of neurotoxin, hemagglutinin, and nontoxic nonhemagglutinin. The three genes encoding these proteins were closely linked on a plasmid of about 114kb (76MDa) but not on chromosomal DNA. Type G is unusual among Clostridium botulinum strains and is physiologically characterized by weak or delayed proteolytic activity, absence of lipase and lecithinase, inability to ferment sugars, infrequent sporulation, and poor production of toxins. The Type G toxin is also produced by Clostridium argentinense.
Clostridium botulinum infection:
Botulism is a fatal type of food poisoning that results from the ingestion of food containing the preformed toxin Clostridium botulinum. In botulism, toxin is absorbed in the gut, then carried by blood to motor nerves. The toxin blocks acetylcholine at neuromuscular junctions. The “4 Ds” of botulism include dysphagia (difficulty in swallowing), diplopia (double vision), dysarthria (difficulty articulating or hoarseness), and dry mouth.
Acute flaccid paralysis – a clinical hallmark of botulism. It begins with bilateral cranial nerve impairment involving muscle of the face, head and pharynx and then descends symmetrically to involve muscles of the thorax and extremities.
Different forms of botulism:
1. Food–borne botulism – is a severe disease with a high fatality rate, not due to an infection of type A strains. It can be caused by consumption of contaminated food in which neurotoxin has been produced. This is usually the result of poor food processing and/or poor temperature control of processed foods, allowing the bacterium to grow anaerobically and so produce toxin in amounts that become harmful.
2. Infant botulism – an infant can acquire botulism by ingesting Clostridium botulinum spores, which are found in soil or honey products. The spores germinate into bacteria that colonize the bowel and synthesize toxins. As the toxin is absorbed, it irreversibly binds to acetylcholine receptors on motor nerve terminals at neuromuscular junctions.
3. Wound botulism – most cases occur in drug injectors, particularly among those using black tar heroin in conjunction with skin popping (subcutaneous and inadvertent intradermal injection). Symptoms of wound botulism often overlap with the symptoms of opioid intoxication or neurologic syndromes such as Guillain-Barré syndrome.
4. Adult intestinal toxemia botulism – has been aptly described as “an elusive disease to classify.” It shares an etiology with infant botulism. Both infant botulism and adult intestinal toxemia botulism are intestinal toxemias, or toxicoinfections, with BoNT–producing clostridia colonizing the intestinal tract and producing botulinum toxin in situ.
5. Iatrogenic botulism – can be due to excessive dosage of botulinum toxin for cosmetic or medical reasons or its frequency in less time. This happens if toxin enters the vascular space and gets through to peripheral cholinergic nerve terminals.
6. Inhalation botulism – Clostridium botulinum neurotoxin serotype A (BoNT/A) is an inhalation poison, and that poisoning is due to the active process of binding and transcytosis across airway epithelial cells rather than paracellular movement.
Administration of botulinum antitoxin is the only
available treatment for botulism. It can arrest the progression of paralysis
and decrease its duration. Botulinum antitoxin can only target the toxins at
the extracellular level and antitoxin treatment cannot reverse the paralysis in
the long–term exposure.
Culture
1. CDC anaerobe blood agar – produces good growth.
2. Phenylethyl Alcohol Agar with 5% Sheep Blood – growth after 1 – 2 days incubation
3. Egg yolk agar – for wound abscess species
a. Modified McClung Toabe agar
b. Lombard–Dowell agar
c. Neomycin Egg Yolk (NEY)
(1) Neomycin is added to achieve a final concentration of 100 ug/ml. Neomycin is heat stable and can also be added to anaerobe blood agar in the same concentration.
(2) Neomycin inhibits some of the facultatively anaerobic gram–negative bacilli, thus NEY is moderately selective.
(3) After incubation, examine the blood agar and PEA with a dissecting microscope for hemolysis pattern, cooling structure, and evidence of swarming organism of motile colonies.
NEY culture has evidence of
(1) Lecithinase – a phospholipase indicated insoluble, opaque, whitish precipitate within the agar.
(2) Lipase activity – indicated by an iridescent sheen or oil on water appearance (pearly layer) on the surface.
(3) Proteolysis – indicated by a zone of translucent clearing in the medium around the colony.
CLOSTRIDIUM DIFFICILE
Characteristics:
1. They are anaerobic, spore–forming, toxin–producing bacillus.
2. Clostridium difficile is the most common cause of nosocomial infections in the United States, surpassing methicillin resistant Staphylococcus aureus.
3. Clostridium difficile infection (CDI) can cause systemic complications including ascites, pleural effusion, cardiopulmonary arrest, hepatic abscess, abdominal compartment syndrome, acute respiratory distress syndrome, and renal failure.
4. Besides antibiotic consumption, the main risk factors associated with the development of CDI are advanced age, impairment in humoral immunity, renal disease, hypoalbuminemia. The clinical manifestations associated with CDI range from asymptomatic colonization and mild diarrhea to toxic megacolon and life-threatening fulminant colitis.
5. They are well suited in the anaerobic environment of the colon, and the presence of glycine and cholate derivatives facilitates the germination of spores.
6. Cycloserine–Cefoxitin–Fructose agar (CCFA) is a selective media for Clostridium difficile.
Clostridium difficile infection (CDI):
Clostridium difficile causes Antibiotic–Associated Diarrhea (AAD) and more serious intestinal conditions such as colitis and pseudomembranous colitis in humans. These conditions generally result from overgrowth of Clostridium difficile in the colon, usually after the normal intestinal microbiota flora has been disturbed by antimicrobial chemotherapy. People in good health usually do not get Clostridium difficile disease.
Toxins produced by Clostridium difficile:
Both toxins (TcdA and TcdB ) cause cells to die through cytopathic effect or cytotoxic effect. Both toxins have a number of effects on cells that do not necessarily result in rounding or death yet may contribute to pathogenesis. Inactivation of Rho GTPases by both toxins results in the disruption of cell–cell junctions, which may contribute to the increased epithelial permeability and luminal fluid accumulation associated with CDAD.
1. Toxin A (TcdA) – referred to as enterotoxin because it causes fluid accumulation in the bowel. It induces a strong inflammatory response, which contributes to the intestinal lesion.
2. Toxin B (TcdB) – is an extremely lethal cytopathic toxin and highly related to TcsL.
3. Clostridium difficile Transferase Toxin (CDT) – unlike TcdA and TcdB which are both single–chain, CDT is a binary actin–ADP–ribosylating toxin that causes depolymerization of actin, thereby inducing formation of the microtubule-based protrusions. It is homologous to the Iota Toxin (ITX) of Clostridium perfringens.
CLOSTRIDIUM SORDELLII
1. It is an anaerobic gram–positive rod with subterminal spores and peritrichous flagella.
2. The infection is considered to result from childbirth, abortion, and/or gynecological procedures. It has been associated with an increased incidence of disease in intravenous heroin users.
3. Although most strains of Clostridium sordellii are nonpathogenic, some virulent, toxin-producing strains cause fatal infections related to their production of exotoxins that cause toxic shock syndrome, even when there are minimal signs of tissue infection.
4. Identification has been successful from clinical specimens via traditional culture, mass spectrometry methods, and molecular methods.
5. The infection begins once the bacteria enter the bodies of patients through trauma, operations, or procedures such as episiotomy and proliferates in low-oxygen environments, such as foci of devascularized tissue.
6. Although infections are relatively rare in humans, the high mortality rate associated with these infections makes it important for these pathogens to be identified.
Toxins produced by Clostridium sordellii:
The toxins produced are believed to be responsible for vascular permeability, refractory hypotension, and edema.
1. Lethal toxin (TcsL) – is causally involved in enteritis of domestic animals and in wound infections of humans. Interestingly, TcsL cytopathic effects increase at low pH, a characteristic found in the vaginal tract. It is a crucial factor involved in the pathogenesis of toxic shock syndrome during infection.
2. Hemorrhagic toxin (TcsH) – not well characterized, but it has been reported to cause dermal and intestinal necrosis in guinea pigs.
3. Sordellilysin (SDL) – a cholesterol–dependent cytolysin (CDC)
THE LISTERIA GROUP
Listeria species are isolated from a diversity of environmental sources, including soil, water, effluents, a large variety of foods, and the feces of humans and animals. The natural habitat of these bacteria is thought to be decomposing plant matter, in which they live as saprophytes.
The genus Listeria currently includes six species: Listeria monocytogenes, Listeria ivanovii, Listeria seeligeri, Listeria innocua, Listeria welshimeri, and Listeria grayi. The first two is pathogenic.
Listeria
ivanovii, a second pathogenic species of the genus, is specific for ruminants.
LISTERIA MONOCYTOGENES
Characteristics:
1. They are Gram–positive, catalase–positive rods, facultatively anaerobic, non–spore forming bacteria showing a typical diphtheroid palisade.
2. Motility at room temperature is called tumbling motility but rarely at 37oC. The motility differentiates Listeria from Diphtheroid.
3. Named after the Scientist Joseph Lister and Monocytes and discovered in 1926 during an epidemic of rabbits and guinea pigs.
4. The major source of human infection is food contaminated with the pathogen, including raw milk and milk products.
5. The unusual tolerance of the bacterium to sodium chloride and sodium nitrite, and the ability to multiply (albeit slowly) at refrigeration temperatures makes Listeria monocytogenes of particular concern as a post–processing contaminant in long–shelf–life refrigerated foods. The widespread distribution of Listeria monocytogenes and the ability to survive on dry and moist surfaces favours post–processing contamination of foods from both raw product and factory sites.
Listeria monocytogenes Infection:
The clinical signs of Listeria monocytogenes infection are very similar in all susceptible hosts. Two basic forms of presentation can be distinguished: perinatal listeriosis and listeriosis in the adult patient. In both instances, the predominant clinical forms correspond to disseminated infection or to local infection in the central nervous system (CNS).
1. Listeriosis – foodborne listeriosis has three main clinical features, namely, meningitis, septicemia, and abortion. In healthy humans it can cause febrile gastroenteritis, but in susceptible persons (children, elderly, immune-compromised and pregnant women) it may lead to septicemia and meningitis. Listeriosis can also manifest as a febrile gastroenteritis syndrome. Listeriosis is usually a very severe disease. It is one of the deadliest bacterial infections currently known with a mean mortality rate in humans of 20 to 30% or higher despite early antibiotic treatment.
2. Listeria monocytogenes Meningoencephalitis (LMM) – presents with fever, neck stiffness, headache, altered mental status, neurological deficits, and other classic signs of meningitis.
3. Granulomatosis infantiseptica – refers to a granulomatous, or inflammatory, nodular rash of the skin caused by a Listeria infection in a newborn child. Occurs when a pregnant individual is infected with Listeria monocytogenes because the individual eats contaminated food.
4. Cutaneous Listeriosis – cutaneous infections due to Listeria monocytogenes are rare. Typically, infections manifest as nonpainful, nonpruritic, localized, papulopustular or vesiculopustular eruptions in healthy persons. The papulopustular or papulovesicular rash that occurs is most often self–limited, and full recovery without antibiotic treatment is usual. This form occurs sporadically among farmers and veterinarians and is contracted by direct contact with the genital tract or placenta from cows having had a miscarriage due to Listeria infection.
5. Ocular Listeriosis is rare, with conjunctivitis being the most frequent manifestation. Keratitis, endophthalmitis, and acute chorioretinitis have also been reported.
6. Listeria infection leads to the production of cold agglutinin for human and sheep red cells as well as specific agglutinating antibody.
Toxins produced by Listeria monocytogenes:
1. Listeriolysin O (LLO) – is a secreted pore–forming protein essential for the escape of Listeria monocytogenes from the vacuole formed upon initial internalization. It is similar in function and antigenicity to streptolysin O (SLO) from Streptococcus pyogenes.
2. Internalin (Inla) – mediates bacterial adhesion and invasion of epithelial cells in the human intestine through specific interaction with its host cell receptor E–cadherin.
3. Phospholipase C – Listeria monocytogenes secretes two distinct phospholipases C, a phosphatidylinositol–specific phospholipase C (PI–PLC) and a broad–range phospholipase C (PC–PLC). Both have role in the initial invasion of host to cell–to–cell spread. PC–PLC is also known as lecithinase.
4. Bile
Salt Hydrolase (BSH)
– a virulence factor involved in the intestinal and hepatic phases of
listeriosis. It catalyses the hydrolysis of the amide bond, liberating the
glycine/taurine moiety from the side chain of the steroid core.
Laboratory diagnosis:
1. Bacterial Culture using the following medium:
a. Al–Zoreky–Sandine Listeria Medium (ASLM) was formulated to recover Listeria monocytogenes from food specimens. The medium contains acriflavine, ceftazidime and moxalactam as selective agents. Gives a positive esculin reaction indicated by black discoloration of the medium.
b. PALCAM agar – highly selective due to inclusion of polymyxin B, acriflavine hydrochloride, lithium chloride, ceftazidime, and acriflavine hydrochloride. A double indicator system is used as the medium contains esculin and ferrous ions as well as mannitol and phenol red. Listeria hydrolyses esculin producing colonies surrounded by a black zone. Listeria is mannitol negative.
c. Listeria Oxford Agar – contains the selective inhibitory components lithium chloride, acriflavine, colistin, Fosfomycin, cefotetan and cycloheximide and (2) the indicator system esculin and ferric iron.
d. McBride Listeria Agar (MLA) – uses phenyl ethanol agar base, lithium chloride, glycine, and blood. Phenyl ethanol and lithium chloride are inhibitory to gram negative organisms. However, it was observed that lithium chloride had a marked inhibitory effect on Listeria monocytogenes, and glycine was also found to be inhibitory to the organism. It was also observed that phenyl ethanol appeared to inhibit the repair of heat-injured cells of Listeria monocytogenes.
e. Lithium Chloride Phenylethanol Moxolactam Agar (LPMA) – similar in composition to MLA except there is a ten–fold increase in the lithium chloride concentration, and glycine anhydride is substituted for glycine because the latter was found to inhibit Listeria monocytogenes. Blood is omitted from this formula and moxalactam, a β–lactam antibiotic effective against a wide range of organisms is added. LPMA is very selective and inhibits contaminants commonly present in Brie cheese, cabbage, and hams.
f. Modified Vogel Johnson Agar (MVJA) – contains potassium tellurite, bacitracin, nalidixic acid, and moxalactam.
g. Dominguez Rodriguez Isolation Agar (DRIA) – by adding esculin as the sole carbohydrate source, and ammonium ferric citrate as an indicator of hydrolysis to a medium containing nalidixic acid and acriflavine, colonies of Listeria monocytogenes could be differentiated with relative ease from contaminants. The color of DRIA turns dark around colonies of Listeria monocytogenes due to the organism's ability to hydrolyse esculin. The hydrolytic product reacts with ammonium ferric citrate to produce the dark color.
h. Trypaflavine Nalidixic Acid Serum Agar (TNSA) – trypaflavine is added to a medium containing nalidixic acid and serum.
i. Modified Despierres Medium (MDA) – useful for enumerating Listeria monocytogenes in cabbage. Incorporates methylene blue into a selective broth containing nalidixic acid and Polymyxin B. The medium inhibited the growth of Streptococcus faecalis.
2. Fluorescence Sandwich Immunoassay based on the use of chitosan–cellulose nanocrystal (CNC) membrane to capture the p60 protein–specific antigen with the use of monoclonal anti–PepD. Labelling is done using Alex Fluor 488.
3. Anton’s Test – done by placing 1 – 2 drops of bacterial suspension into the eyes of the rabbit or guinea pig. Positive result is a purulent conjunctivitis develops into the inoculated eye after 24 – 36 hours if organisms are Listeria monocytogenes.
4. Cold Enrichment Technique – proven useful for isolating Listeria monocytogenes from highly contaminated samples such as feces, vegetation, raw milk, and soft and hard cheeses.
5. Henry Oblique Transillumination (HOT) Technique – in this method, presumptive Listeria monocytogenes colonies are viewed through a dissecting microscope. The light source for the microscope is reflected from a mirror positioned to reflect the light on the bottom of the petri dish at a 45o angle. The colonies will appear pearlescent blue when observed in this manner, and several workers have used this method to identify Listeria in heavily contaminated backgrounds.
6. Simplified Henry Oblique Transillumination (SHOT) Technique – for ease of viewing and counting (scoring) of suspect colonies, each agar plate was examined by placing it bottom side up on a transparent platform that was tilted towards the viewer at 45o and illuminated directly from below with a high–intensity lamp at a right angle (90o) to the bench top. Each colony on the agar plate was scanned with a 5x magnifying hand lens attached to a tube that was clamped at 45o with the aid of set square, thereby attaining the angle of transillumination of 135o. The agar plates were freed of water condensate to reduce distortion.
7. Gram staining
8. Gene
probes and Monoclonal antibody
THE ERYSIPELOTHRIX INSIDIOSA aka ERYSIPELOTHRIX RHUSIOPATHIAE
Characteristics:
1. They resemble diptheroids.
2. They are zoonotic organisms. Infection is especially common among individuals who handle fish. Most cases in humans and other animals probably occur via scratches or puncture wounds of the skin but in some cases, it appears that the organism has penetrated intact skin.
3. It causes three major forms of disease in humans: erysipeloid (localized cutaneous infection), diffuse cutaneous infection, and systemic infection (bacteremia with or without endocarditis).
4. They demonstrate pleomorphism except those isolated from tissues.
5. They are thin, microaerophilic, non–encapsulated, nonsporulating, non–motile, gram–positive rods.
6. They are catalase and oxidase negative oftentimes arranged singly, in short chains, or in pairs in a "V" configuration or are group randomly. Filaments and long chains are sometimes seen. Growth is improved by 5 to 10% carbon dioxide.
Cultural media:
1. Erysipelothrix Selective Broth (ESB) – containing tryptose broth base, 5% horse serum, and antibiotics such as kanamycin (400 μg/ml), Neomycin (50 μg/ml), and vancomycin (25 μg/ml).
2. Schaedler or Trypticase soy broth
3. Heart Infusion agar, Blood agar plate, Chocolate Agar Plate – alpha– hemolytic and colonies are barely visible for at least 48 hours and described as lampbrush, test tube brush.
4. Tellurite medium – black colonies
5. TSI – H2S positive but catalase negative
THE MYCOBACTERIA GROUP
Myco refers to the fungus–like properties of the mycobacterium which grows in long branches and grows slowly.
General characteristics:
1. They are gram positive, aerobic, non–encapsulated, non–motile, non–sporeformers. They are slow–growing and free–living in soil and water.
2. They possess a large amount of lipids on their cell wall. Mycolic acid is a cell wall lipid that is unique to Mycobacteria and is a key virulence factor.
3. They multiply by binary fission.
4. They contain Much granules which are polyphosphate in nature.
5. They are also called ACID FAST BACILLI because stain is not easily removed with acidified solvent, therefore they are acid resistant. They appear as slender, filamentous rods which are colored red against a blue background.
6. In fluorescent stained smears, they appear as red orange fluorescent with rhodamine and auramine.
7. Infections from the most pathogenic specie are mostly due to inhalation of droplet nuclei. Most of the species produce skin and soft tissue infection (SSTI) and lymphadenitis.
8. A Mycobacterial capsule consists of neutral polysaccharides, proteins, and lower amounts of lipids. It was determined that polysaccharides are the major capsular components in slow–growing mycobacterial species while proteins seem to be the main constituents in fast–growers.
MYCOBACTERIUM LEPRAE
Characteristics:
1. Also known as Hansen’s bacillus. They are nonmotile and microaerophilic.
2. It is acid fast bacilli found inside modified mononuclear or epithelioid structures producing lepra cells which are found in palisade or parallel formation, suggesting cigar packet.
3. Typical cells are found predominantly in smears and scrapings obtained from the skin and mucous membrane of patients with leprosy.
4. Ability to oxidize DOPA (3,4–dihydroxyphenylalanine) as compared with other Mycobacteria. However, another study mentioned that hyaluronic acid and not Mycobacterium leprae is responsible for DOPA oxidation, and phenolase activity is not associated with the metabolism of Mycobacterium leprae.
5. Mycobacterium leprae parasitizes histiocytes (skin macrophages) and Schwann cells in the peripheral nerves.
6. Glycine in the cell wall components differs from other AFB.
7. Specific antigen is phenolic glycolipid (PGL–I). The unique triglycosyl unit of PGL-I of Mycobacterium leprae contains methylated glucopyranose (Glcp) and Rhap residues in 3,6-di-O-methyl-β-d-Glcp-(1→4)-2,3-di-O-methyl-α-l-Rhap-(1→4)-3-O-methyl-α-l-Rhap.
8. The Mycobacterium leprae cell wall includes more mycolic acid than that of Mycobacterium tuberculosis.
9. Treatment with pyridine causes rapid loss of acid fastness.
10. The organism is unculturable on artificial media or human tissue culture cells, but they can be grown in food pads of mice. The armadillo is susceptible to leprosy and has been used experimentally. Feet, upper, lip, external ear, hip, tail, and genitals are inoculated simultaneously. Commonly used mice are nude mice, nude rat, lasat mouse.
Mycobacterium leprae infection
It is responsible for leprosy, a chronic communicable disease characterized by lesions of the skin – infiltration, macules, papules, and nodules and by involvement of peripheral nerves with muscle weakness and paralysis and trophic changes in skin, muscle, and bone. Macrophages have an important role in leprosy pathogenesis mediating interactions between the host and the bacteria.
Leprosy has been classified into five types using the Ridley–Jopling classification:
a. Tuberculoid
(TT)
b. Borderline
tuberculoid (BT)
c. Mid-borderline
(BB)
d. Borderline
lepromatous (BL)
e. Lepromatous (LL)
Classification according to Center for Disease Control (CDC):
a. Multibacillary (MB) or Lepromatous or nodular type – the course is progressive and malign, with nodular skin lesions, slow, symmetric nerve involvement, presence of abundant acid-fast bacilli.
b. Paucibacillary (PB) or Tuberculoid or anesthetic type – the course is benign and non–progressive with macular skin lesions, severe asymmetric nerve involvement of sudden onset, few acid-fast bacilli.
c. Borderline or dimorphous type – it is of intermediate severity. The skin lesion seems to be of the tuberculoid type, but are more numerous, and may be found anywhere on the body. Peripheral nerves are affected as well, with ensuing weakness and anesthesia.
A new specie, Mycobacterium lepromatosis was discovered in 2008 and causes diffuse lepromatous leprosy (DLL), a unique form of leprosy endemic in Mexico. DLL is characterized clinically by diffuse, non–nodular cutaneous infiltration, manifesting recurrent crops of large sharply demarcated skin lesions called Lucio’s phenomenon, which is considered as an unusual reaction to the infection. In the advanced stage, the lesions may become ulcerated, particularly on the lower extremities, or even generalized, leading to fatal secondary bacterial infection and sepsis. Mycobacterium lepromatosis infection is associated with more variation of skin lesions and biopsy sites than is Mycobacterium leprae infection.
|
Mycobacterium
leprae |
Mycobacterium
lepromatosis |
Laboratory diagnosis
1. Ziehl–Nielsen technique – using skin or nasal scrapings or skin earlobe biopsy.
2. Fite–Faraco staining – combines peanut oil with the deparaffinizing solvent (xylene) to minimize the exposure of the bacteria's cell wall to organic solvents thus protecting the precarious acid-fastness of the organism. One of the flaws of the procedure is the density of the bacilli should be about 1000 per cubic millimeter of the tissue to pick single bacilli in the section.
3. Fluorescent Microscopy using Auramine Rhodamine Stain
4. Harada’s staining – employs periodic acid–carbol pararosaniline and periodic acid–methenamine silver stain for demonstrating chromophobic bacilli which do not get stained with conventional carbol fuchsin or counter stain.
5. Polymerase Chain Reaction – this technology has come to be used not only for diagnosis, but also for detection of resistant bacteria, identification of the route of infection, evaluation of therapeutic effects, and confirmation of household contacts. It has been verified and reported that multiplex real-time quantitative PCR (qPCR) using two or three genes, namely RLEP, 16S rRNA and superoxide dismutase (sodA), is effective.
6. Immunoblot and ELISA–based studies of Mycobacterium leprae antigens (PGL–I) are useful for diagnosis and evaluation of the effectiveness of antibiotic treatment. Can be done using CSF or urine samples.
7. Lepromin test – a diagnostic skin test for leprosy that measures an individual’s capability to develop cell–mediated immunity to Mycobacterium leprae. It has limited practical use because it does not indicate exposure. It involves the intradermal injection of 40 million bacilli / ml heat-killed Mycobacterium leprae derived from infected armadillos. The injection site is evaluated at 1–2 days and 3 weeks.
Two types of reaction
a. Early or Fernandez reaction – occurs within the first 2 days and represents a delayed–type hypersensitivity reaction.
b. Late or Mitsuda reaction – occurs after 3 – 4 weeks. A positive Mitsuda reaction is described as an indurated lesion of more than 4 mm that histologically shows granuloma formation.
MYCOBACTERIUM TUBERCULOSIS
Characteristics:
1. Also known as Koch’s bacillus.
2. Stained by carbol fuchsin (hot staining), fluorochrome dye like rhodamine auramine and they appear slender, beaded rods producing singly X, Y, V & L.
3. They are obligate aerobe with increase carbon dioxide tension.
4. They are also slow growers and appear after 2 – 3 weeks incubation at 35 – 37oC.
5. It has no toxins, resistant to heat, drying and chemical agents such as acid and alkali due to hydrophobic nature of organism.
6. Glycerol is used as the standard carbon source to grow bacteria.
7. They are fastidious organisms requiring enriched media and 5 – 10% CO2 for growth.
8. They can survive in the host for prolonged periods of time without inducing any symptoms and its capability to switch between the asymptomatic non–infectious phase and a clinically apparent infectious phase.
9. Cord factor (trehalose 6,6’–dimycolate or TDM) is a surface glycolipid, which causes MTB to grow in vitro in serpentine cords. Cord factor is an inhibitor of PMN (Polymorphonuclear neutrophil) migration and is also toxic to mammalian cells.
10. Multidrug–resistant tuberculosis (MDR–TB) indicates resistance to both isoniazid and rifampin.
11. They grow on a complex media containing egg yolk, tissue extract, animal serum, penicillin, or malachite green. Specimen for culture is subject to chemical methods, digestion and decontamination using a mucolytic agent, N–acetyl–L–cysteine (NALC) NaOH, cetylpyridium or trisodium phosphate benzalkonium chloride.
Media used:
1. Agar based medium
a. Oleic acid albumin medium – both used for diagnostic test and for determining streptomycin resistance.
b. Middle Brook 7H9 liquid medium
(1) Positive
result is the appearance of breadcrumb–like lumps without turbidity.
(2) When
combined with tetrazolium bromide provides better result.
(3) A modified formulation was used for Microscopic Observation Drug Susceptibility (MODS).
2. Egg based medium
a. Petragnani
medium
b. ATS
(American Trudeau Society medium)
c. LJ
(Lowenstein Jensen medium)
d. Dorset
egg medium
e. Stonebrink
Medium – for Mycobacterium bovis
Types of tubercle bacilli:
1. Human and bovine strains – pathogenic for man either respiratory or gastrointestinal route
Mycobacterium tuberculosis or Koch’s bacillus is the human strain and Mycobacterium bovis, the bovine strain.
2. Atypical mycobacteria – MOTT (Mycobacteria other than tubercle bacilli) or Runyon’s group – causes tuberculosis like disease in man.
|
|
Human |
Bovine |
|
Colony |
flat, irregular margin, dry, friable, rough, warty, granular &
buff color with cauliflower appearance |
pyramidal colony |
|
Growth |
Eugonic – easily detached from the medium but difficult to emulsify |
Dysgonic – difficult to detach from the medium but easily emulsified |
|
Niacin Test |
Positive |
Negative |
Clinical manifestation of TB:
1. Low grade fever, night sweats and chronic weight loss. As the disease progresses, a cough develops with the production of sputum and hemoptysis.
2. TB is primarily a pulmonary disease, but many organs (extrapulmonary) maybe attacked:
a. Pott’s
disease – spinal disease of TB
b. Lupus
vulgaris – skin disease of TB
c. Scrofula
– cervical lymph node disease of TB
Types of infection:
1. Primary – or the initial phase, occurs in people without specific immunity, generally normal children and young adults who have not previously been exposed to Mycobacterium tuberculosis. The initial infection can occur at any time during childhood, but adolescence is the peak time of risk. Primary disease develops within 5 years of the initial infection, which stimulates specific immunity, demonstrated by the development of a positive skin response to purified protein derivative of tuberculin.
2. Reactivation – caused by tubercle bacilli which has survived from the primary lesion or newly inhaled organism from the environment.
When an individual has first contact with tubercle bacilli, an acute exudative lesion develops and spreads rapidly to the lymphatics and regional lymph nodes. The “Ghon complex” (usually in the lungs) is the primary tissue lesion together with the involved lymph nodes. The lesion heals rapidly, and the lymph node undergoes caseation which usually calcifies. The tuberculin test becomes positive.
3. Latent
Tuberculosis Infection (LTBI) – is defined by the World Health
Organization as a state of persistent immune response to stimulation by
Mycobacterium tuberculosis antigens with no evidence of clinically manifest
active TB.
Laboratory diagnosis
1. Gram stain
a. Mycobacteria vary from gram (+) to “gram ghost” or “gram neutral” bacilli.
b. The large amount of lipids present in the cell walls of mycobacteria renders them impermeable to the dyes used in gram stain.
2. AFB stain
a. Carbol fuchsin phenol
When phenol is added to the staining solution, such water–soluble basic dyes behave in effect like their lipid–soluble counterparts. The loss of mycobacterial acid–fastness with carbol-fuchsin after bromination or chromation indicates that this phenomenon is related to the presence of unsaturated lipids in the bacterial cells.
b. Flurochrome stain
(1) Examples are auramine O and rhodamine B in combination with phenol.
(2) Detects non–viable organism especially when TB bacilli are killed using treatment with isoniazid.
3. Concentration technique
a. This is a digestion–decontamination procedure because most specimens for mycobacterial culture contain large number of organic debris and various organisms that rapidly outgrown mycobacteria.
b. Action of these substances
(1) They dissolve fats and mucus thereby freeing bacteria.
(2) They kill all other organisms in the specimen except Mycobacterium tuberculosis.
4. Virulence test
a. Serpentine cord formation – taken from the specimen’s tube where condensed water is found. It is then smeared, stained, and must observe the serpentine cord formation.
b. Neutral dye test
Based on the capacity of Mycobacterium tuberculosis to synthesize phthiocerol dimycocerosates (DIM/PDIM), a cell wall methyl–branched lipid that has been related to virulence of the organism.
Organism + 50% methyl alcohol, then incubate at 37oC for one hour, decant the alcohol. The organism from sediments is then treated with fresh bacterial buffer and neutral red dye solution. Allow to incubate for 1 hour at 37oC and observe for red coloration.
5. Tuberculin test – is a hypersensitivity test for tuberculosis.
a. It is a model for the study of cell–mediated immunity.
b. It illustrates the concept of anergy (a confirmed tuberculosis with repeatedly negative tuberculin test)
c. It illustrates the concept that infection does not necessarily mean a disease.
Infection means the presence of tubercle
bacilli in the body sufficient to produce the delayed type of hypersensitivity
of a positive tuberculin test.
Disease means the presence of the clinical manifestations of tuberculosis such as abnormal chest X–rays.
Conversion of the tuberculin test from a negative to a positive one is useful for the diagnosis of recent tuberculosis infection.
Types of Tuberculin Test:
a. Old tuberculin – a concentrated filtrate of broth in which the tubercle bacilli have been grown for six weeks.
b. Purified Protein derivative (PPD) – a chemical fractionation of OT and it is then prepared material for skin testing. It is a culture filtrate of the organism and purified by protein with TCA.
Positive tuberculin test – does not indicate an active disease.
Tuberculin positive person is at risk of developing disease from reactivation of the primary infection whereas the tuberculin (–) person is not at risk.
Different methods of PPD:
(1) Mantoux test – injection intracutaneously.
(2) von Pirquet test – scratching tuberculin into skin.
(3) Vollmer patch test – soaking a piece of cloth/filter paper in OT/PPD and placing it over the skin.
(4) Moro percutaneous test – OT/PPD treated with anhydrous lanolin ointment and then rub.
(5) Trambusti's Test – dipping the injection needle in a 10% tuberculin solution prior to injection to a depth of 5mm.
(6) Heaf
test
– a multiple puncture apparatus provides automatic punch mechanism whereby six
needles are released to puncture the skin evenly to a depth of 1 or 2 mm.
RUNYON’S CLASSIFICATION OF ATYPICAL MYCOBACTERIA
1. Scotochromogen – cultures whose colonies produce pigment when grown either in the dark or the light.
a. Mycobacterium scrofulaceum – cervical adenitis.
b. Mycobacterium szulgai – “tap water” scotochromogen.
c. Mycobacterium gordonae – least pathogenic and its isolation is typically regarded as a contaminant. The organism is ubiquitous, and it is most isolated from soil and water.
2. Non–photochromogen – cultures whose colonies are nonpigmented or possess only pale shades of pigment and whose color is unaffected by exposure to light.
a. Mycobacterium avium complex or MAC – consists of multiple nontuberculosis mycobacterial (NTM) species, which cannot be distinguished in the microbiology laboratory and requires genetic testing. They are nonmotile, non–spore–forming, gram–positive acid–fast bacillus and take about 10 to 20 days to develop mature colonies.
(1) Mycobacterium
avium
(2) Mycobacterium
intracellulare (Battey bacillus)
(3) Mycobacterium chimaera
b. Mycobacterium xenopi – pulmonary disease
c. Mycobacterium
terrae
– “radish” bacilli, associated with tenosynovitis.
Mycobacterium triviale – “V” bacillus, recovered from tuberculous infection.
d. Mycobacterium gastri – “J” bacillus, isolated from gastric washing.
e. Mycobacterium ulcerans – the third most common mycobacterial infection after leprosy and tuberculosis in immunocompetent people. It causes Buruli ulcer which is a chronic debilitating disease that mainly affects the skin and sometimes bones.
f. Mycobacterium haemophilum – known to cause cutaneous and subcutaneous infections, septic arthritis, osteomyelitis, and pneumonitis in immunocompromised patients. Cervicofacial lymphadenitis is the most common manifestation in immunocompetent children. The source of infection is from water reservoirs. It also appears to be pathogenic in fish and has caused clinical manifestations in snakes and bison similar to those seen in humans.
3. Photochromogen – cultures whose colonies are nonpigmented when grown in the dark but become pigmented after they are exposed to light for one or more hours.
a. Mycobacterium kansasii – pulmonary tuberculosis
b. Mycobacterium marinum – given the name “swimming pool” granuloma. It produces cutaneous disease manifesting as a solitary papulonodular lesion on a finger or hand. Superficial infection produces multiple nodules resembling sporotrichosis. Occasionally, skin lesions appear as pustular, nodulo–ulcerative, granulomatous, or verrucous plaques. Deep infections, such as tenosynovitis (the most frequent), osteomyelitis, arthritis, and bursitis, occur in 20 to 40% of the cases.
c. Mycobacterium simiae – transmitted by inhalation of aerosols or by inoculation. This organism can be found in municipal water sources, as well as soil, salt, foodstuff, and even air samples. It has been identified in hospital drinking fountains, sinks, and ice machines and can act as a reservoir for nosocomial Mycobacterium simiae outbreaks. It can also contaminate medical equipment and laboratory specimens.
d. Mycobacterium asiaticum – reported to be responsible for extrapulmonary disease in humans, namely, flexor tenosynovitis, olecranon bursitis, and keratitis. The most important reservoir is the potable water supplies although there is seasonal variations in the occurrence which is attributed to the change in water temperature.
4. Rapid Growers Mycobacteria (RGM)
a. Mycobacterium smegmatis – disseminated infections are commonly related to immunosuppression. Clinical infections are often found to be a result of contaminated surgical or other wounds, caused using contaminated solutions or lipid creams that act as carriers of the disease, as well as exit site infections, tunnel or pocket infections, and catheter–related infections.
b. Mycobacterium phlei – it is rod–shaped and pleiomorphic and can exist in a coccoid form under certain environmental conditions. The coccoid form represented a resting stage in aging cultures as suggested by “Time lapse” microscopy but when exposed to fresh media these coccoid forms reverted to rod–shaped bacteria. It is nonpathogenic but most infections are related to the spine.
c. Mycobacterium vaccae – not considered a human pathogen, however, some reports indicate it as lung and cutaneous pathogen.
d. Mycobacterium fortuitum – is frequently associated with skin, soft tissue, and bone infections while it rarely causes pulmonary or disseminated diseases. Skin and soft tissue infections are common after mammoplasty and similar plastic surgery interventions or cardiac surgery (sternal wound infections). Foot baths of pedicure salons have also been identified as a source of infection associated with furunculosis.
e. Mycobacterium chelonae – causes catheter–related infections and post–surgical infections after implants, transplants, and injections such as sclerotherapy. The eye is the second most frequent organ involved. Invasive infections like bacteremia, osteomyelitis, intraabdominal abscess, and disseminated cutaneous infections are common in immunosuppressed patients such as those on steroids, monoclonal antibodies, and post-transplant immunosuppression.
f. Mycobacterium abscessus complex (MABC) – a group of rapidly growing, multidrug–resistant, nontuberculous mycobacteria that are responsible for a wide spectrum of skin and soft tissue diseases, central nervous system infections, bacteremia, and ocular and other infections. They are resistant to disinfectants and, therefore, can cause postsurgical and postprocedural infections. They are especially prevalent in respiratory specimens from patients with cystic fibrosis. MABC is differentiated into three subspecies:
(1) Mycobacterium
abscessus subspecie abscessus – causes pulmonary infection.
(2) Mycobacterium
abscessus subspecie massiliense – causes otitis media.
(3) Mycobacterium abscessus subspecie bolletii – pulmonary infection.
Type
1 to 3 are slow growing (>7 days) while Type 4 are fast growers (<7 days)
Procedures useful in differentiating Mycobacteria:
1. Niacin test – depends on the formation of a complex color compound when a pyrimidine compound (niacin) reacts with cyanogen bromide. It is useful in differentiating humans from bovine strains of tubercle bacilli.
2. Nitrate reduction test – only a few species of mycobacteria produce nitroreductase which catalyzes the reduction of inorganic nitrate to nitrite. The development of a red color on addition of sulphanilic acid and N–naphthylethylenediamine to an extract of the unknown culture is indicative of the presence of nitrite and a positive test.
3. Catalase test – the enzyme catalase splits hydrogen peroxide (H2O2) into water and oxygen, which appears as bubbles. The semi–quantitative catalase test detects differences among certain mycobacteria in their production of catalase by measuring the height of the column of bubbles produced after the addition of H2O2, i.e., those species producing < 45 mm of bubbles and those producing > 45 mm of bubbles.
4. Tween 80 hydrolysis – Tween–80 is the trade name of a detergent that can be useful in identifying those mycobacteria that possess a lipase that splits the compound into oleic acid and polyoxyethylated sorbitol. The released oleic acid changes the optical characteristics of the substrate so that the neutral red indicator changes from an original amber color to pink.
5. Sodium chloride tolerance test – few mycobacteria can grow in culture media containing 5% sodium chloride. The exceptions include Mycobacterium triviale and most of the rapid growers except the Mycobacterium chelonae complex.
6. Arylsulfatase test – if appreciable amounts of arylsulfatase have been produced, phenolphthalein will be split from the sulfate and detected by alkalinizing with NaCO3. The resulting color is compared immediately with a set of standards. A positive result shows a range from pink to bright red.
7. Tellurite reduction test – most valuable in separating Mycobacterium avium complex from the saprophytic photochromogen.
8. Growth on McConkey Agar without Crystal Violet – most isolates of the Mycobacterium fortuitum and Mycobacterium chelonae complexes will grow on MacConkey agar without crystal violet, whereas most other rapid growers will not.
9. Urease Test – urease is an enzyme possessed by many Mycobacterium species that can hydrolyse urea to form ammonia and carbon dioxide. The ammonia reacts in solution to form ammonium carbonate, resulting in alkalinization and an increase in the pH of the medium. A color change from amber to pink or red is a positive reaction.
10. Pyrazinamidase Test – the enzyme pyrazinamidase hydrolyses pyrazinamide to pyrazinoic acid. Pyrazinoic acid is detected by the addition of ferrous ammonium sulphate to the culture medium. The formation of a pink ferrous–pyrazinoic acid complex indicates a positive test.
11. Iron Uptake Test – Mycobacterium fortuitum and a few other rapid- and slow-growers can convert ferric ammonium citrate to iron oxide. Iron oxide is visible as a rust color in the colonies when grown in the presence of ferric ammonium citrate.
12. Triophene-2-carboxylic
Acid Hydrazide (TCH) Tolerance Susceptibility Test – TCH
selectively inhibits the growth of Mycobacterium bovis; however, Mycobacterium
tuberculosis and most other slowly growing mycobacteria are resistant to TCH at
levels of 10 μg ml−1 in the medium.
THE CORYNEBACTERIA GROUP
General characteristics:
1. They are gram positive, non–sporulating, non–motile, often club–shaped and frequently banded or beaded with reddish metachromatic granules known as Babes Ernst Bodies, which contain polymerized polyphosphate.
2. They frequently exhibit characteristic arrangement, resembling Chinese letters or palisades.
3. Generally, they are aerobic, or facultative but microaerophilic species do occur. They are catalase positive.
4. Anaerobic organisms formerly in this genus are now placed in the Genus Propionobacterium.
5. Individual cells tend to lie parallel or at an angle to each other, resulting in V, L, or Y shapes due to their snapping movement.
6. Although the appearance of the organism in stained smear is highly characteristic. It should not be identified with morphology alone, many diptheroids and actinomyces stain in the same irregular fashion and pleomorphic.
7. The cell envelope of the family Corynebacterineae is very complex, and this complexity contributes substantially to their virulence. The peptidoglycan layer that surrounds a standard IM contains covalently attached arabinogalactan and this is covalently attached to mycolic acids.
CORYNEBACTERIUM DIPTHERIAE
Characteristics:
1. Corynebacterium diphtheria is anaerobic, non–motile, non–spore–forming, non–capsulated, toxin–producing, pleomorphic coccobacillus, which is usually club–shaped that acts to destroy tissue within the mucous membranes. They are usually catalase positive.
2. On smear, the organisms often have a “pick-up sticks” relationship, assuming parallel (palisade–like), or V– or L–type patterns. The organisms are resistant to environmental changes, such as freezing and drying.
3. Diphtheria predominantly affects children of <15 years of age, and several investigations have shown that unimmunized and immunocompromised populations are particularly vulnerable to the disease.
4. Diphtheria is generally an acute respiratory infection, characterized by the formation of a pseudo membrane in the throat, but cutaneous infections are possible. Systemic effects, such as myocarditis and neuropathy, which are associated with increased fatality risk, are due to diphtheria toxin, an exotoxin produced by the pathogen that inhibits protein synthesis and causes cell death. The toxin can cause damage to the myelin sheaths in the central and peripheral nervous system leading to loss of motor control or sensation.
5. Adhesion in Corynebacterium diphtheriae is mediated mainly by pili (or fimbriae) that are covalently attached to the bacterial cell wall and contribute to colonization in the host.
Classification of Diphtheria Infection:
1. Respiratory – involving the anterior nasal, pharyngeal, and laryngeal cavities and the tonsils. The typical advanced symptoms of acute diphtheria include the presence of a thick, grey layer (called the pseudomembrane) on the throat and/or tonsils, enlarged lymph node glands in the neck (bull neck) and, in severe cases, myocarditis and inflammation of the nerves.
2. Cutaneous – may present as a scaling rash or ulcers with clearly demarcated edges and membrane and may also include the genital area. It is commonly seen in tropical countries.
3. Ocular – result of the primary infection (keratoconjunctivitis) and the release of exotoxin (motility disorders).
Diphtheria Biotypes:
On McLeod’s Potassium Tellurite Blood Agar, it produces three biotypes:
1. Gravis – appear as large, flat, dark, gray, daisy head colony, non–hemolytic, evenly stained. It is the only biotype that ferments starch.
2. Mitis – appears as small, black, glossy, β–hemolytic with many metachromatic granules, convex to translucent with white center like fried egg.
3. Intermedius – grayish black on CTA but colorless on BAP with club ends.
4. Belfanti – grey or black, opaque colonies, entire edge, and glossy smooth surface; size variation is common with small zone of β–hemolysis, non–maltose fermenter. It is the only biotype that is negative for nitrate.
Laboratory Test:
1. Stained smear
Albert’s stain is used for staining of volutin-containing organisms. In Albert’s stain, metachromatic (volutin) granules stain bluish black and bacterial protoplasm green. It involves fixing the smear for a minute with a solution of toluidine blue and malachite green and application of Albert's iodine.
2. Culture
a. Tinsdale medium – Corynebacterium diptheriae form a brown halo which is caused by the action of cysteine sulfhydrolase with the formation of ferric sulfide in the medium.
b. Egg and serum medium – used as a maintenance medium for diphtheria.
c. Cystine Tellurite Blood Agar (CTA) – tellurite inhibits most gram–negative organisms while enhancing the growth of Corynebacterium specie and some other gram–positive bacteria. CTA is preferred than Tinsdale as medium. Tinsdale is useful in differentiating the Corynebacteria.
3. Toxigenicity or Virulence Test
a. In vivo test: Animal inoculation test
(1) Intracutaneous test – 0.1 cc of emulsified bacterial culture is injected into a guinea pig or rabbit in two different areas. After 4 hours, guinea pig or rabbit demonstrates inflammatory necrosis while those protected animals produce no lesions.
(2) Subcutaneous test – 500 u of antitoxin is injected. After 18 – 24 hours, protected animal remains alive, and death occur within 2 – 3 days in those animals that are unprotected.
b. In Vitro Test: Plate Tests
(1) Colony Overlay Test (COT) – involves inoculating material from an isolated colony of Corynebacterium diphtheriae to a small area on the surface of an agar medium which overlays a monolayer of toxin–susceptible HeLa cells. If toxin is produced during incubation at 37oC, it diffuses to the tissue monolayer and destroys the cells below the inoculation site. 24 hours after inoculation, organisms are killed, and tissue cells are fixed with formaldehyde. The agar overlay is then removed, and the monolayer is stained with crystal violet. Toxin-affected areas fail to stain or stain poorly. A second plate with antitoxin incorporated in the overlay serves as a control for specificity.
(2) Plate Virulence Test – the Elek Test works on the principle of antigen and antibody immunoprecipitation. In this assay, a known toxigenic strain (positive control), a non-toxigenic strain (negative control) and the sample strains are inoculated onto Elek agar medium with a paper strip containing DAT (500 IU/ml) placed onto the agar surface.
4. Schick test – a susceptibility test for diphtheria.
Diphtheria toxin is injected intradermally and after 5 days, the site of injection is examined; if no local reaction occurs, Schick test is negative indicating that the patient has sufficient circulating antitoxin to neutralize the toxin injected.
If redness on the area occurs, Schick test is positive, the individual has insufficient antitoxin unless allergic reaction to the toxin has occurred.
Schick test due to allergy can be
detected using a control consisting of heat inactivated toxin.
THE DIPTHEROIDS
Diphtheroids are defined as aerobic, non–sporulating, pleomorphic Gram–positive bacilli which are more uniformly stained than Corynebacterium diphtheriae, lack the metachromatic granules and are arranged in a palisade manner. They are usually commensals of the skin and mucous membranes. They differ from Corynebacterium diphtheriae in biochemical reactions as well as in toxin production. It is also known as “Non–Diphtheria Corynebacterium.” Some of the identified diphtheroid are:
1. Corynebacterium pseudotuberculosis has been shown to cause lymphadenitis in humans and because of the potential to cause zoonotic infections, milk and meat consumers are exposed to greater risk.
2. Corynebacterium pseudodiphtheriticum is part of the oropharyngeal bacterial flora and is therefore associated mainly with respiratory disease like nosocomial pneumonia, tracheitis, bronchitis and a few other infections.
3. Corynebacterium minutissimun has been isolated from superficial skin infections and very rarely from invasive infections.
4. Corynebacterium ulcerans toxigenic strains have been isolated from nasopharyngeal infections.
5. Corynebacterium xerosis species have been found in conjunctival sac and on skin and mucous membranes.
6. Corynebacterium hoffmanni (Hoffmann's bacillus) which is also spoken of as pseudo-diphtheria bacillus, is a common organism in the throats of healthy persons and is found also in cases of diphtheria.
Please
note that the term "Coryneform" and "Diphtheroid" were
arbitrarily used in different research paper and journals during this research.
For educational purposes, both terms were used here as defined by the authors
of the publication. By literal definition, the term "Diphtheroid"
would mean "mini diphtheria" while "Coryneform" would mean
"like Corynebacteria."
THE CORYNEFORM
Coryneform bacteria are characterized as irregularly shaped, non–spore–forming, aerobic, gram–positive rods. Coryneform bacteria are ubiquitous in the environment (soil and water), commensal colonizers of skin and mucous membranes in humans, and commensals in animals. Most coryneform bacteria colonize before infection, and most infections are acquired during hospitalization.
1. The Lipophilic Corynebacteria – they grow poorly in broth or as tiny pinpoint colonies in 24 hours on standard plate agar but grow more luxuriantly if the agar or broth is supplemented with an exogenous lipid such as sterile Tween 80.
a. Corynebacterium jeikeium (formerly “Corynebacterium group JK”) – it has been reported to cause sepsis primarily in patients with neoplastic diseases with associated risk factors, such as prolonged hospitalization, neutropenia, treatment with multiple antibiotics and disruption of the skin surface.
b. Corynebacterium urealyticum – formerly known as CDC coryneform group D2, is multiresistant to antibiotics, and causes bacteriuria mainly in patients hospitalized for long periods or who undergo urological manipulation.
c. Corynebacterium afermentans subsp. lipophilum – most of the isolate originates from abscess materials.
d. Corynebacterium accolens – was first described from human clinical specimens, such as wound drainage, endocervix samples, sputum, and throat swab specimens.
e. Corynebacterium macginleyi – the presence of this species in the normal conjunctiva is not surprising because the meibomian glands produce meibum, an oily substance. It is the most isolated strain in the conjunctiva, and it is also recognized as the most common causative agent of opportunistic ocular infections.
f. Corynebacterium tuberculostearicum – named as such because their fatty acid profile is composed of tuberculostearic acid. It is a major bacterial species that colonize a variety of skin environments, including dry, moist, and sebaceous regions. The disease associated have included inflammatory breast disease, pancreatic panniculitis, chronic rhinosinusitis, and surgical site infections. It is also identified in keratinocyte carcinomas (i.e., basal, and squamous cell carcinomas). It is also isolated from pus from women with mastitis, implicating their possible involvement in cases of inflammatory breast disease.
g. Corynebacterium kroppenstedtii – first isolated in sputum specimen in 1998. It is associated with breast conditions, especially recurrent granulomatous mastitis (GM). Occasional reports of bacteremia and a single report of prosthetic valve infection have also been described. Further study is warranted to confirm its association with psychiatric illnesses.
h. Corynebacterium bovis – colonizes the teat canal and is controlled by post milking teat antisepsis in cows. They produce small, white, powdery, or granular colonies after 48 hours. The organism has a requirement for lipids and tends to grow best in the primary portion of the streak.
i. Corynebacterium lipophiloflavum – isolated from a patient with bacterial vaginosis.
j. Corynebacterium kutscheri – it has been described as a commensal bacterium in mice, rats, and voles and has been identified in the oral cavity, esophagus, colon, rectum, and submaxillary lymph nodes of these rodents. It was first called pseudotuberculosis, as clinical disease with the organism resembled mouse tuberculosis, with pulmonary abscesses and caseating necrosis.
k. Corynebacterium resistens – causes fatal bacteremia in immuncompromised patients in Japan and known to be multi–drug resistant to antimicrobials. On TSA with 5% sheep blood, they produce nonpigmented, greyish–white, glistening, pearly colonies up to 1 mm in diameter. Colonies were catalase positive, oxidase negative, nonhemolytic, and very slow growing under anaerobic conditions. Tween 80 enhances growth, resulting in colonies 2 to 4 mm in diameter but CAMP negative.
l. Corynebacterium ureicelerivorans – is an opportunistic pathogen with a lipophilic lifestyle and an exceptionally high urease activity.
2. The Non–lipophilic Fermentative Corynebacteria – uses a substrate such as lactic acid, acetic acid, and propionic acid in the formation of volatile fatty acids (VFA).
a. Corynebacterium ulcerans
b. Corynebacterium pseudotuberculosis
c. Corynebacterium xerosis
d. Corynebacterium striatum – is part of the normal flora of the nostrils and skin. This organism has been increasingly reported as the etiological agent of various human infections, including bacteraemia and fatal pleuropulmonary infection, endocarditis, meningitis, cerebrospinal fluid shunt infections in children, conjunctivitis, chorioamnionitis and peritonitis.
e. Corynebacterium minutissimum – is the causative agent of erythrasma. This cutaneous infection manifests as well–demarcated, reddish brown patches and plaques located in moist intertriginous zones. Infections usually occur after puberty.
f. Corynebacterium amycolatum – it has been isolated from urine, pus, catheter tips, blood, prostatic secretion, cerebrospinal fluid, and sputum thus associated with septicaemia, endocarditis, meningitis, septic arthritis, and urinary tract infections.
g. Corynebacterium glucuronolyticum – is a rare species isolated from male patients with genitourinary tract infections, probably being part of the normal male genitourinary microbiota, while its presence in females is uncertain.
h. Corynebacterium argentoratense – it is isolated from the throats of patients. It is characterized by the presence of chemotype IV, a cell wall, corynomycolic acids, and a G+C content ranging from 60 to 61 mol%.
i. Corynebacterium matruchotii (previously Bacterionema matruchotii) – has long filaments and a typical “wipe-handle” morphology. It is part of the core tooth microbiota in humans. It colonizes the oral cavity, the skin, and likely the nose and nasopharynx, but is not detected in the gastrointestinal tract or human milk.
j. Corynebacterium riegelii – isolated from females with urinary tract infections. Cells are gram positive, non–spore forming, and nonmotile. They are typically club–shaped rods which appear as single cells, in pairs, or in small clusters. Colonies are whitish, circular with entire edges, convex, glistening, of up to 1.5 mm in diameter after 48 hours of incubation, and of a creamy or a slightly sticky consistency.
k. Corynebacterium
confusum
– has been isolated from a blood culture, foot infections, and breast abscess.
Cells are Gram–positive, non–spore–forming and non–motile. They are typically
club–shaped rods that occur as single cells, in pairs or in small clusters.
THE PROPIONIBACTERIUM
They are a non–spore–forming, anaerobic, pleomorphic rod whose end products of fermentation include propionic acid. The organism belongs to the human cutaneous Propionibacteria, along with Propionibacterium avidum, Propionibacterium granulosum, Propionibacterium innocuum, Propionibacterium modestum. All species are now known as Cutibacterium.
PROPIONIBACTERIUM ACNES
Characteristics:
1. Now known as Cutibacterium acnes.
2. They could form biofilms which aids in micro-colony formation, evasion of macrophage engulfment, avoidance of phagocytosis.
3. Propionibacterium acnes grows slowly and can resist phagocytosis and persist intracellularly within macrophages. This resistance to phagocytosis may be conferred by a complex cell wall structure, which also includes a surface fibrillar layer.
4. The essential oil of Litsea cubeba has recently been found to be effective in the treatment of acne vulgaris.
5. They can be isolated using Thioglycolate broth and Schaedler Agar Media.
6. Propionibacterium acnes is ubiquitous on human skin, preferably within sebaceous follicles, where it is generally a harmless commensal. Nevertheless, it is an opportunistic pathogen. It has been isolated from sites of infection and inflammation ranging from acne to diverse other conditions such as corneal ulcers, endocarditis, pulmonary angiitis, hyperostosis, endophthalmitis, sarcoidosis, sciatica, and osteitis, SAPHO (synovitis, acne, pustulosis, hyperostosis, osteitis) syndrome as well as prosthetic joint infection (PJIs).
7. Three subspecies has been identified:
Cutibacterium acnes subspecies acnes (type I) – associated with acne.
Cutibacterium acnes subspecies defendens (type II) – associated with healthy skin or deep tissue infections.
Cutibacterium acnes subspecies elongatum (type III) – associated with healthy skin or deep tissue infections.
Virulence Factor:
1. CAMP Factor – is an essential source of inflammation in acne vulgaris. The CAMP factor acts as a pore–forming toxin that may induce cytolysis and cytokine secretion via activation of the inflammasome.
2. Lipase – secreted by this microorganism metabolizes sebum and the resulting metabolites evoke inflammation in human skin.
3. Porphyrin – are group of
metabolites essential to the biosynthesis of heme, cobalamin, and chlorophyll
in living organisms. Bacterial porphyrins are proinflammatory, with high levels
linked to human inflammatory diseases, including the common skin condition acne
vulgaris. Propionibacteria are among the most abundant skin bacteria.
Variations in Propionibacteria composition on the skin may lead to different
porphyrin levels and inflammatory potentials. High levels of porphyrin
production by this microorganism have been linked to acne severity.
Other Pathogenic Species:
1. Propionibacterium avidum causes to a limited extent, periprosthetic joint infection (PJI). They are often isolated from moist areas of the skin such as the nares, axilla, rectum, and groin.
2. Propionibacterium granulosum can cause infections in patients compromised by recent surgery, trauma, or implanted devices (e.g., prosthetic heart valves and cerebrospinal fluid shunts).
3. Propionibacterium humerusii (now Cutibacterium modestum) is a clinical isolate recovered from the humeral membrane of a patient who underwent revision of a failed total shoulder arthroplasty.
THE FILAMENTOUS BACILLI
Both Actinomyces and Nocardia has been known as a “bacteria that masquerade Fungi.” They both cause similar clinical syndromes involving the lung, bone and joint, soft tissue, and the central nervous system. The hallmark of both infections is abscess formation and chronic progression of infection without regard to anatomic barriers. Patients frequently present with fistulous tracts and draining sinuses.
1. Actinomycetes are Gram positive, aerobic, indole negative and filamentous bacteria. They exhibit filamentous branching structures which then fragment into bacillary or coccoid forms. Several members of the Actinomycetes are well known for their distinctive capacity to secrete secondary metabolites having biological properties like antimicrobial, anti–parasitic, antioxidant, anticancer and anti–biofouling etc.
Human actinomycosis is a chronic granulomatous infectious disease caused by Actinomyces species. Cervicofacial actinomycosis is the most frequent clinical form of actinomycosis, and “lumpy jaw syndrome”, which is associated with odontogenic infection, is the most common clinical manifestation, representing approximately 60% of all reported cases.
a. Actinomyces israelli is a filamentous anaerobic Gram–positive bacterium that is part of the normal bacterial flora of the oral cavity and upper gastrointestinal tract. Histologically, the long filamentous organisms stain dark blue on routine hematoxylin and eosin preparations and are particularly easy to recognize when associated with characteristic sulfur granules. It is one of the most common species involved in abdominal actinomycosis and IUD infection.
b. Actinomyces turicensis are normal commensals of the oral, gut, vagina, and skin flora. Infection with these organisms is usually benign, and bacteremia is rare. It has also been implicated in appendicitis and is frequently accompanied by aerobic bacterial isolates of the Streptococcus anginosus group.
c. Actinomyces europaeus is a facultatively anaerobic, gram-positive filamentous bacillus that has been a well–documented culprit of abscesses, decubitus ulcers, and urinary tract infections in the past. It is also a primary causal agent of necrotizing fasciitis.
d. Actinomyces odontylicus is an endogenous infection arising from the mucous membranes. It causes thoracopulmonary infections and cervicothoracic disease in immunocompromised patients.
e. Tropheryma whipplei causes a disease known as Whipple's disease, which is characterized by intestinal malabsorption leading to cachexia and death. When first discovered by George Whipple, he described a “silver-stained rod-shaped microorganisms” in the vacuoles of macrophages of affected patients, he did not think of them as the cause for the disease.
2. Nocardiae are aerobic, gram–positive bacilli present mainly in soil. The genus Nocardia are gram–positive organisms that are partially acid fast due to the mycolic acid content of the cell wall.
Pulmonary disease is the most common clinical presentation of Nocardia infection, accounting for more than 40% of reported cases.
Lymphocutaneous nocardiosis is sometimes referred to as “sporotrichoid–type” disease owing to its similar appearance to cutaneous sporotrichosis. This Nocardia infection is marked by the presence of a primary pyodermatous lesion frequently associated with areas of chronic drainage and crusting. In contrast to primary cutaneous disease, the organism invades more deeply to involve the lymphatic system and progresses to the formation of lymphatic abscesses.
a. Nocardia asteroides is filamentous and may be visible in an H&E stain, but it is Grocott–positive and variably acid–fast using the modified ZN for leprosy. However, it is difficult to demonstrate even with the acid–fast bacillus techniques. They present as a systemic illness but can represent a rare cause of back pain secondary to involvement of the bony spinal column. Nocardia usually spreads hematogenously from a pulmonary focus to the soft organ systems, but osteomyelitis has also been reported on rare occasions. There is a higher incidence of cervical and thoracic osseous involvement. This is related to the proximity of the pulmonary focus. Spread occurs through venous drainage of the retropharyngeal and mediastinal sites.
b. Nocardia transvalensis causes Mycetoma pedis or Madura foot. It is characterized by a combination of painless subcutaneous mass, multiple sinuses and discharge containing grains. Within the grain, the causative agent is found. It usually spreads to involve the skin, deep structures, and bone, resulting in destruction, deformity, and loss of function, which may be fatal. Mycetoma commonly involves the extremities, back and gluteal region but any other part of the body can be affected.
c. Nocardia farcinica infections are rare and potentially life threatening. Associated with pulmonary disease such as pneumoconiosis.
d. Nocardia brasiliensis are responsible for most cases of Nocardia pneumonia but also causes endophthalmitis.
OTHER GRAM–POSITIVE BACILLI
1. Kurthia gibsonii can spread from an animal to a human by zoophilic sexual intercourse, and the mucous membranes of the human genital tract can support bacterial survival.
2. Rothia dentocariosa is a nonmotile, non–sporulating, non–acid–fast, gram–positive bacillus with a variable morphology that includes coccoid and branching filamentous forms. Colonies are either smooth and convex or rough with a “spoke–wheel” surface with scalloped edges.
3. Eggerthella lenta (formerly Eubacterium lentum) is a normal human microflora that is anaerobic, non–sporulating, and Gram positive. It was previously reported to cause endocarditis.
4. Brevibacterium is characterized by non–spore–forming, nonmotile, catalase–positive Gram–positive rods. They are found in raw milk and surface–ripened cheese as well as on human skin and in animal sources. It is mainly found in habitat that has high salt concentration. Brevibacterium genus is a heterogeneous group of bacteria that are coryneform & diphtheroid. Patients with indwelling central venous catheters are at high risk of acquiring bloodstream infections. Some of the pathogenic species includes Brevibacterium luteolum, Brevibacterium sanguinis, and Brevibacterium casei.












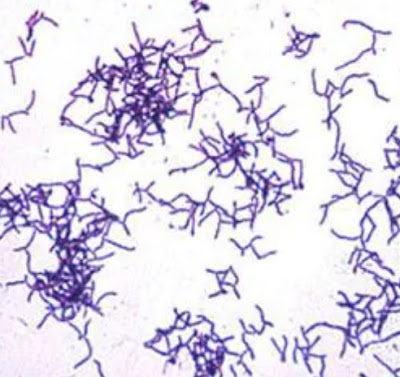

No comments:
Post a Comment