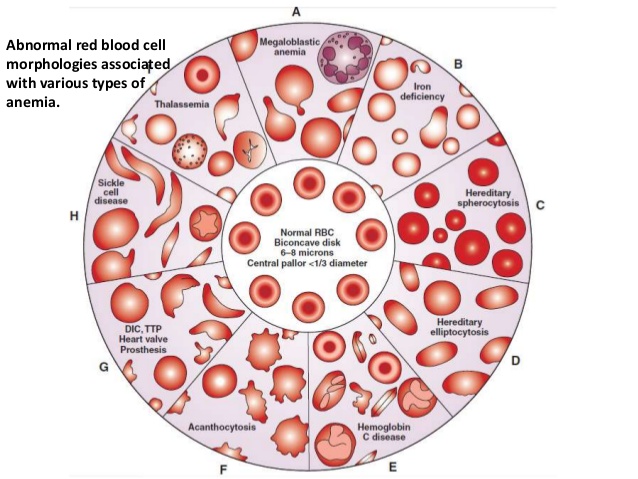
ANEMIA
Anemia means an
impoverished condition of the blood caused by reduction in red blood cell,
hemoglobin or both. It is considered to be present if the hemoglobin
concentration or the hematocrit is below the lower limit of the 95% reference
range interval for the individual’s age, sex and geographic location
(altitude).
Clinical signs of
anemia
Clinical signs and
symptoms result from the diminished delivery of oxygen to the tissues and,
therefore, are related to the lowered hemoglobin concentration and blood
volume, and dependent upon the rate of these changes.
In general, the anemic
patient complains of easy fatigability and dyspnea and exertion, and often of
faintness, vertigo, palpitations and headache. The more common physical
findings are pallor, a rapid, bounding pulse, low blood pressure, slight
fevers, some dependent edema and systolic murmurs. In addition to these general
signs and symptoms, certain clinical findings are characteristic of the
specific type of anemia.
Morphologic
classification of anemia
1. Macrocytic
normochromic anemia – the red blood
cells are larger than normal but contain only the normal amount of hemoglobin.
MCV is greater than 96 fl. MCHC is normal.
a. Megaloblastic
anemia – this is a group of anemias
in which the erythroblasts in the bone marrow show a characteristic
abnormality, maturation of the nucleus being delayed relative to that of the
cytoplasm. The nuclear chromatin maintains and open, stippled, lacey appearance
despite normal hemoglobin formation in the erythroblasts as they mature.
Causes:
(1) Vitamin B12 deficiency – pernicious anemia
(2) Folic acid deficiency – nutritional megaloblastic
anemia
(3) Abnormalities of Vitamin B12 or folate
metabolism
(4) Inherited disorders of DNA synthesis
(5) Drug induced disorders of DNA synthesis
Laboratory
findings
(1) RBC is macrocytic – MCV is greater than 95 fl. and
often as high as 120 – 140 fl.
(2) Macrocytes are typically oval in shape.
(3) Reticulocyte count is low in relation to the degree of
anemia
(4) Total white cell count and platelet counts may be
moderately reduced, especially in severely anemic patients
(5) A proportion of the neutrophils show hypersegmented
nuclei.
(6) Bone marrow is usually hypercellular and the
erythroblasts are large and show failure of nuclear maturation maintaining an
open, fine, primitive pattern but normal hemoglobinization.
b. Non–megaloblastic
anemia – this group of anemia is
characterized by macrocytosis wherein the bone marrow shows normoblastic rather
than megaloblastic erythropoiesis.
Causes
(1) Accelerated erythropoiesis
(2) Increased membrane surface area
(3) Obscure causes, e.g. hypoplastic and aplastic anemias
(4) Alcohol
(5) Liver disease
(6) Cytotoxic drugs
Laboratory
findings
(1) MCV is rarely above 110 fl
(2) Oval macrocytes are seldom seen
(3) Macropolycytes are absent
(4) Bone marrow erythropoiesis is normoblastic
macronormoblastic.
2. Hypochromic
microcytic anemia – the red blood
cells are smaller than normal and contain small amount of hemoglobin. MCV is
less than 80 fl. and MCHC is less than 32%
Causes
a. Iron deficiency
b. Disorder of globin synthesis as in thalassemia
c. Disorder of porphyrin and heme synthesis as in
sideroblastic anemia
d. Other disorders of iron metabolism
Laboratory
findings
a. Microcytes and hypochromic cells are present
b. MCH and MCV is reduced, MCHC is decreased
c. Blood smears show leptocytes, sideroblasts,
siderocytes, poikilocytes
3. Normochromic
normocytic anemia – the red blood
cells are of normal size and contain normal amount of hemoglobin. MCV is 80 –
96 fl. MCHC is normal.
Causes:
a. Recent blood loss
b. Overexpansion of plasma volume as in pregnancy
c. Hemolytic diseases
d. Hypoplastic bone marrow, e.g. aplastic anemia
e. Infiltrated bone marrow, e.g. leukemia
f.
Endocrine
abnormality
g. Chronic disorder
h. Renal disorder
i.
Liver diseases
Laboratory
findings:
a. Plasma volume and
red cell volume – reduced in proportionate amount
b. Hematocrit is
normal
c. Platelet count is
reduced
d. Plasma fibrinogen
level is reduced
e. Neutrophilic
leukocytosis is present
f. Normocytes and
normochomic cells is present
Pathogenic
classification of anemia
1. Anemia due
to blood loss – post–hemorrhagic
anemia
a. Acute
post–hemorrhagic anemia – if blood is
lost over a short period of time in amounts sufficient to cause anemia.
After
a single episode of bleeding, the major manifestations are those due to
depletion of blood volume (hypovolemia). This type of anemia is usually
normocytic. The peripheral blood smear show normal red blood cells in
morphology despite the drop of hemoglobin, RBC count and hematocrit. An
elevated reticulocyte count in the absence of a normal bilirubin would suggest
increased bone marrow activity, without increased red cell or hemoglobin
breakdown.
b. Chronic
post–hemorrhagic anemia – if blood is
lost in small amounts over an extended period of time, lacking both the
clinical and hematologic features that characterize acute post–hemorrhagic
anemia are lacking.
The
reticulocyte count may be normal or slightly increase. Significant anemia does
not usually develop until after storage iron is depleted; the anemia,
therefore, is one of iron deficiency. The anemia is at first normochromic and
normocytic and gradually the newly formed red cells become microcytic, then
hypochromic. The WBC count is normal or slightly decreased, platelet count is
commonly increased and only later, in severe iron deficiency is likely to be
decreased.
2. Anemia due
to accelerated red cell destruction –
hemolytic anemia
Hemolytic
anemia maybe due to:
a. A defect
of the red cell itself – intrinsic hemolytic
anemia. These are usually hereditary and are commonly grouped as membrane,
metabolic or hemoglobin defects.
(1)
Due to
membrane disorders
(a)
Hereditary
spherocytosis
This
is the most common hereditary hemolytic anemia in North Europeans, probably due
to one or other of a variety of defects in a structural protein (spectrin) of
the red cell membrane. The marrow produces red cells of normal biconcave shape
but these lose membrane as they circulate through the spleen and the rest of
the RES. The ratio of the surface area to volume decreases and the cells become
more spherical and ultimately are unable to pass through the splenic
circulation where the spherocytes die prematurely.
Laboratory
findings:
·
Osmotic fragility
test is increased
·
Reticulocytes are
usually 5 – 20%
·
Blood film shows
microspherocytosis
·
Direct Coombs
(antiglobulin) test is negative
·
MCV is normal, MCHC
is often increased
(b)
Paroxysmal
Nocturnal Hemoglobinuria or Marchiava–Michelle syndrome
This
is a rare acquired defect of the red cell membrane which renders it sensitive
to lysis by complement causing chronic intravascular hemolysis.
Typical
nocturnal or sleep–related hemoglobinuria occurs during sleeping or immediately
after awakening. Here, the red cells are thought to be sensitive to a lowering
of the pH of the plasma. This occurs during depressed respiration while
sleeping, caused by the retention of carbon dioxide resulting in acidosis.
Laboratory findings:
· Positive Sucrose
Hemolysis test or Ham’s Acidified Serum Test
· WBC count and
platelet count are often low
· Hemosiderinuria
is a feature
· Reticulocyte
count is lower in relation to degree of anemia
(c)
Hereditary
Elliptocytosis
Much
less is known about this abnormality than hereditary spherocytosis. The disease
is inherited as a dominant characteristic and has been associated with severe
hemolytic anemia in infants.
Laboratory findings:
· Non–hypochromic
elliptocytes are abundant on blood film.
(d)
Hereditary
Pyropoikilocytosis
This
is a rare, moderately severe congenital hemolytic anemia characterize by
microcytosis, striking micropoikilocytosis and fragmentation and autosomal
recessive inheritance. It occurs primarily in Blacks.
(e)
Hereditary
Stomatocytosis
This
is a rare congenital anemia inherited as recessive autosomal pattern. Ten to
thirty percent of red cells show a mouth like linear pallor instead of the
normal central round pale area. Osmotic fragility test is increased.
(f)
Hereditary
Acanthocytosis
This
is caused by an absence of beta–lipoprotein and produces the characteristic
acanthocyte. The condition is associated with plasma lipid abnormalities,
including low total lipid, cholesterol and phospholipid. Marked autohemolysis
occurs, which is enhanced in the presence of EDTA.
(2)
Due to
hemoglobin disorders
(a)
Sickle
cell anemia
Homozygous
HbS disease, a form of hemoloytic anemia which is also hereditary occurs almost
in the Black race. A pathological Hb, known as HbS is responsible for the
conversion of normal erythrocytes to sickle cell. The HbS is insoluble and
forms cyrstals when exposed to low oxygen tension, the red cells sickle and may
block different areas of the microcirculation causing infarcts of various
organs, the abnormality is due to substitution of valine for glutamic acid in
position 6 in the beta chain. In this homozygous form of the disease, the HbS –
HbS molecule is inherited from both parents.
Sickle cell trait – a
heterozygous HbS disease which is a benign asymptomatic condition. The HbS
molecule is inherited from either father or mother. No hematological
abnormalities are found except for the electrophoretic pattern of HbS and HbA.
Laboratory findings:
Sickle cell anemia
· Anemia is
normochromic and normocytic
· Polychromasia is
increased; normoblasts are present
· Target cells are
numerous, Howell – Jolly bodies are seen
· Osmotic fragility
test is decreased
· Mechanical
fragility test is increased
· Positive for
Sickling test – Metabisulfite Test or Dithionite Tube Test
· Hb
electrophoresis at pH 8.4 = HbS constitute over 80% on HbA, HbF 1 – 20%
Sickle cell trait
· Hb
electrophoresis = HbA = 50 to 65%; HbS = 35 to 45%
· Hematuria may
occur
· No anemia with
normal presence of red cells
(b)
Thalassemias – comprise a heterogenous group of hereditary
disorders of hemoglobin synthesis in person of Mediterranean, African and Asian
ancestry. The common characteristic of these disorders is impaired production
of polypeptide chains of hemoglobin; that is the rate of synthesis is diminished
but the chain is, in most cases, structurally normal.
Homozygous Beta Thalassemia – Thalassemia major, Cooley’s anemia,
Mediterranean Anemia, Target cell anemia
In
this type of thalassemia, the beta chain production is decreased. The anemia is
hypochromic and microcytic. Extreme poikilocytosis with bizarre shapes, target
cell, ovalocytosis, Cabot rings, Howell–Jolly bodies, nuclear fragments,
siderocytes, anisochromia, anisocytosis and often extreme normoblastosis are
present. Osmotic fragility is decreased.
Heterozygous Beta Thalassemia – Thalassemia minor, Cooley’s trate,
Rietti–Greppi–Micheli disease
This
is an asymptomatic illness with mild or no anemia, but with prominent
morphologic abnormalities of erythrocytes. The thalassemia gene is inherited
from either father or mother. On stained films, the cells have a moderate
degree of microcytosis and poikilocytosis; target cells and basophilic
stippling are often present. Osmotic fragility is decreased.
Alpha thalassemia – although
this condition is associated with the lack of alpha chain production, the
nature of the genetic defect is still not completely known. Forms of alpha
thalassemia are Hydrops fetalis and HbH disease.
(c)
Hereditary
persistence of Fetal Hemoglobin F
A
group of conditions with HbF production persisting beyond infancy without
significant hematologic abnormalities. It is found in about 0.1% of American
Blacks.
(d)
Other
forms of hemoglobinopathies
Double
heterozygous for two beta chain abnormalities
Double
heterozygous for beta hemoglobinopathy and beta thalassemia
(3)
Due to
metabolic disorders
(a)
Glucose–6–phosphate
dehydrogenase deficiency
It
is a complex heterogenous disorder which is ubiquitous and is the most common
defect seen in the enzyme deficient hemolytic anemias. This is usually
associated with sensitivity to certain drugs, sulfonamide, aspirin, primaquine
and to ingestion or inhalation of the pollen of the common European broad bean
(Vicia fava), thus the disorder is called Favism.
Laboratory
findings:
· The laboratory
findings during active hemolysis are those of hemolytic anemia in general. In
the blood film, poikilocytes, some spherocytes and irregularity contracted
cells are seen.
· Heinz bodies may
be present early in acute hemolytic episode.
· Positive in Dye
Reduction Test or Motulsky or Ascorbate Cyanide Test or in Fluorescent Spot
Test
· Findings can be
confirmed with quantitative assay of G–6–PD
(b)
Pyruvate
kinase deficiency
This
is the most common red cell enzyme deficiency involving the Embden – Meyerhoff
glycolytic pathway. PK deficiency results in a mild to moderately severe
hemolytic anemia with splenomegaly
Laboratory
findings:
· Blood film may
show no notable red cell abnormalities until after splenectomy, when
echinocytes, irregularly contracted red cells, and crenated red cells may be
prominent
· Reticulocyte
count is elevated
· Positive in
Fluorescent Spot Test
· Findings can be confirmed
by quantitative assay of PK
(c)
Pyrimidine–5–nucleotidase
(PN) deficiency
This
is probably one of the more common enzyme deficiencies responsible for
hereditary hemolytic anemia
Acquired
PN deficiency occurs in lead poisoning and is probably responsible for the
basophilic stippling in that condition.
Laboratory findings:
· Marked basophilic
stippling in red cells
· Reticulocytosis
is observed
· Positive in the
demonstration of decreased nucleotidase activities
b. A factor
outside the red cell and action upon it
– extrinsic hemolytic anemias. These are almost always acquired. Causes:
(1)
Chemical agents –
drugs, chemicals
(2)
Physical agents –
heat, trauma
(3)
Vegetable and
animal poison
(4)
Infectious agents
– malarial parasite, bacteria
(5)
Presence of
autoantibodies, isoantibodies or drug–related antibodies – causes:
Immune
Hemolytic Anemias
Immune
hemolytic anemias are disorders in which erythrocyte survival is reduced
because of the deposition of immunoglobulin and/or complement on the red cell
membrane. The immune hemolytic anemias can be grouped according to the presence
of autoantibodies, isoantibodies, or drug–related antibodies
(a)
Autoimmune
Hemolytic Anemia
Autoimmune
Hemolytic Anemia is due to an altered immune response resulting in the
production of antibody against the host’s own erythrocytes, with subsequent
hemolysis. The AIHA can be classified according to serologic or clinical
characteristics:
(1)
AIHA
associated with warm antibody – AIHA
is mediated by antibody with maximum binding affinity at 37oC.
(2)
AIHA
associated with cold antibody – AIHA
is mediated by antibody with maximum binding affinity at 4oC.
Paroxysmal
Cold Hemoglobinuria – this is a rare
state in which hemolysis occurs when blood is warmed after previous exposure to
chilling. Exposure of the hands and feet to cooling and then subsequent warming
will often be sufficient to produce hemolysis. The anemia is caused by the
presence of any autohemolysin in the plasma that becomes attached to the red
cell in the cold. When the red cells are warmed, this antibody causes lysis in
the presence of complement.
The
antibody (Donath – Landsteiner antibody) present if the IgG form and manifests
itself clinically by muscular aches, back pain, diarrhea, weakness, transient
chills and hemoglobinuria. The acute form may follow an acute viral illness,
but the chronic form is associated with congenital syphilis.
Laboratory findings:
· Elevated
reticulocyte count
· Increased
concentration of indirect bilirubin
· Hemoglobinuria
· Positive Donath –
Landsteiner or Rosenbach or Ehrlich or Sanford method
(b)
Isoimmune
Hemolytic Anemia
Isoimmune
hemolytic anemia usually occurs in newborns following the transplacental
passage of maternal anti – fetal red cell antibody
(1) Isoimmune hemolytic disease of the newborn due to Rh
incompatibility – Erythroblastosis fetalis
(2) Isoimmune hemolytic disease of the newborn due to ABO
incompatibility
(c)
Drug–induced
Immune Hemolytic Anemia
Immune
hemolytic anemia may occur following the administration of drugs.
Four
mechanisms appear to mediate the immune hemolysis.
(1)
Adsorption of
Immune Complexes to red cell membrane
(2)
Adsorption of
drug to red cell membrane
(3)
Induction of
autoantibody by drugs
(4)
Non–immunologic
adsorption of immunoglobulin to red cell membrane
3. Anemia due
to impaired red cell production
a. Deficiency
of essential substance
(1)
Iron,
folic acid, Vitamin B12
Iron
deficiency anemia
Iron
deficiency results only when there is an increased need for iron (e.g., during
rapid growth in infancy or during pregnancy) or when excessive loss of blood
has reduced the body’s reserve of iron (e.g., following repeated hemorrhages,
excessive menstruation or multiple pregnancies).
Laboratory
findings:
· In early iron
deficiency anemia, the stained blood film often shows normochromic normocytic
erythrocytes
· In later stages,
the blood picture is one of microcytosis, anisocytosis, poikilocytosis and
varying degrees of hypochromia
· Reticulocytes are
decreased
· MCV, Hb and Hct
are low
· Serum iron level
is reduced and the serum total iron binding capacity is increased
Folic
acid deficiency anemia
This
type of anemia manifests a macrocytic megaloblastic type similar in morphology
to pernicious anemia. The usual causes are poor dietary intake of folic acid,
disordered absorption in the small intestine, increased consumption during
pregnancy, and antagonism between drugs and folic acid.
Laboratory
findings:
·
Similar with
pernicious anemia
Pernicious
anemia or Vitamin B12 deficiency anemia or Addison’s anemia
Anemia
caused by maturation failure of erythrocytes due to Vitamin B12
deficiency. The usual causes of the deficiency are poor absorption of Vitamin B12,
inadequate oral intake, defective production of intrinsic factor, and
interference with intestinal absorption.
Laboratory
findings:
· Reduction in
hemoglobin, RBC count, hematocrit and corresponding alterations in red cell
indices
· Peripheral blood
smear shows a moderate to marked degree of macrocytosis, anisocytosis,
poikilocytosis, basophilic stippling and nucleated red cells
· Granulocyte often
shows enlargement and multilobulation, being termed “macropolycytes” or “P.A.”
poly cells
· Bone marrow is
hypercellular and is dominated by the presence of megaloblastic anemia
(2)
Protein
deficiency – malnutrition, kwashiorkor
(3)
Possibly
ascorbic acid, copper, cobalt, nickel
b. Deficiency
of erythroblasts
(1)
Aplastic
anemia
Aplastic
(hypoplastic) anemia is defined as pancytopenia (anemia, leukemia and
thrombocytopenia) resulting from aplasia of the bone marrow. It is classified
into primary types which include a congenital form (Fanconi anemia) and
acquired form with no obvious precipitating cause. Secondary aplastic anemia
may result from ionizing radiation, chemicals, drugs, viral infection.
Pancytopenia
refers to a disorder in all three blood forming series of cells of the bone
marrow – red blood cell, white blood cell, platelets. All these cells are
reduced in number.
Laboratory
findings:
· RBC is
normochromic, normocytic or macrocytic
· Reticulocyte
count is reduced
· Leukopenia and
thrombocytopenia are present
· There are no
abnormal cells in the peripheral blood
· Bone marrow shows
hypoplasia
(2)
Pure Red
Cell Aplasia
This
is a rare syndrome characterized by anemia with normal leukocytes and platelets
and grossly reduced or absent erythroblasts from the marrow.
(a)
Congenital
Red Cell Aplasia or Diamond–Blackfan Anemia
This
is a rare, constitutional red cell aplasia which usually becomes obvious during
the first year of life by may occur as late as six years of age.
(b)
Acquired
Pure Red Cell Aplasia
In
middle aged adults, selective failure of red cell production occurs rarely.
Laboratory findings
· Reticulocyte
count is low
· Leukocyte and
platelet counts are normal
· Marrow shows
reduction in all developing erythroid cells except pronormoblasts
c. Infiltration
of the bone marrow
Myelopthisic
anemia – anemia is associated with
bone marrow infiltration. This anemia is associated with marrow replacement by
(or involvement with) metastatic carcinoma, multiple myeloma, leukemia,
lymphoma, lipidoses or storage disease and certain other conditions.
Laboratory
findings
· The
characteristic finding is the presence of varying numbers of normoblasts and
immature neutrophils; these are responsible for the descriptive terms
leucoerythroblastic reaction, leucoerythroblastic anemia and
leukoerythroblastosis.
· Normochromic and
normocytic (occasionally macrocytic) anemia is present
· Reticulocytes are
increased and the number of normoblasts is usually out of proportion to the
severity of anemia
· WBC count is
normal or reduced or occasionally elevated
· Platelet count is
normal or decreased and bizarre, atypical platelets are sometimes seen
· Immature
neutrophils and myeloblasts may be found
d. Sideroblastic
anemia
This
is a refractory anemia with hypochromic cells in the peripheral blood and
increased marrow iron with many pathological ring sideroblasts present. These
are abnormal erythroblasts containing iron granules arranged in a ring or collar
around the nucleus instead of the few randomly distributed iron granules seen
when normal erythroblasts are stained for iron.
(1)
Hereditary
sideroblastic anemia – this is
characterized by a markedly hypochromic and microcytic blood picture. This is
due to a congenital enzyme defect, e.g., of delta amino–levulinic acid
synthetase or heme synthetase.
(2)
Primary
acquired sideroblastic anemia – this
occurs in either sex mainly in middle or old age and is due to a somatic
mutation of the erythroid progenitor cells causing not only defects in heme
synthesis but also defects in DNA synthesis with megaloblastic and other
dyserythropoeitic features and frequently a raised MCV.
(3)
Sideroblastic
anemia associated with other disorders
like acute myeloid leukemia, erythroleukemia, myeloma
(4)
Secondary
sideroblastic anemia – this occurs in
the bone marrow of patients taking certain drugs, excess alcohol or with lead
poisoning
e. Anemia of
chronic disorder
This
is the anemia most commonly seen in chronic infections, rheumatoid arthritis
and neoplastic disease. This is usually mild and is overshadowed by the basic
disease. usually, the anemia does not progress in severity and has
characteristic morphologic, biochemically kinetic disturbances.
f. Anemia in
liver disease
This
is anemia associated with liver disease as in liver cirrhosis and others
g. Anemia in
endocrine disease
Anemia
associated with diseases of the endocrine glands, like hypothyroidism,
pituitary deficiency and others.
h. Anemia of
renal insufficiency
Anemia
associated with chronic renal failure, hemolytic uremic syndrome and others.
i. Congenital
dyserythropoietic anemia (CDA)
Hereditary
anemia characterized by abnormal erythropoiesis with ineffective erythropoiesis
and splenomegaly
LABORATORY DIAGNOSIS
OF ANEMIA
The diagnosis and study of
anemia required the proper use and interpretation of laboratory
measurements. Prerequisites for the
efficient use of the laboratory are a careful history and physical examination,
both of which lead to the initial laboratory measurements and provide important
guidance in determining the nature of anemia.
Basic examination
includes the following:
1.
Hemoglobin
determination
2.
Erythrocyte count
3.
Reticulocyte
count
4.
Leukocyte count
5.
Platelet count
6.
Hematocrit
determination
7.
Differential
leukocyte count
8.
Examination of
the blood film
9.
Red cell indices
10. Erythrocyte Sedimentation Rate
After the basic
examination, the choice of further procedures depends upon the type of anemia
as determined by the indices, blood film and clinical findings.
Special tests used in
further investigation of anemia:
1. Bone
marrow aspirate
2. Erythrocyte
Survival Studies – this is valuable
in the diagnosis of hemolytic anemias
Radioactive
chromium (51Cr) is convenient and widely used. Labeled chromate is
added to a blood sample in vitro and binds to beta chains of hemoglobin. The
chromated red cells are injected intravenously and their disappearance is
measured by counting blood which is sampled every 1 to 2 days for 10 days to 14
days. Residual activity is an index of the intravascular life span of the
labeled red cells. Since 51Cr emits gamma rays, external scanning
can detect sites of red cell destruction.
The
erythrocyte life span is usually expressed as the period during which one half
of the radioactivity remains in the blood. Chromium normally elutes from the
red cells at a rate of 1% per day. Thus, the half life of the 51Cr–labeled
erythrocytes in normal individuals is 25 to 32 days instead of 60 days.
3. Osmotic
Fragility Test
The
osmotic fragility test determines the fragility of red cells when placed in a
series of serially diluted hypotonic saline solutions. It measures the
resistance of the red cells to hemolysis by osmotic stress. Normal red cells
when placed in hypotonic salt solutions absorb fluid, thus causing the volume
to increase and the shape to change from that of biconcave discs to spherical
forms. Further expansion of volume leads to cell rupture or hemolysis. When red
cells are placed in hypertonic solution, they lose fluid and crenate.
For
testing the osmotic fragility of red cells, they are suspended in a series of
tubes containing hypotonic solutions of NaCl varying from 0.9 to 0.0%,
incubated at room temperature for 30 minutes and centrifuged. The percent hemolysis
in the supernatant solutions is measured and plotted for each NaCl
concentration. Cells which are thicker than normal or more spherical, like the
spherocytes, with decreased surface / volume ratio, have a limited capacity.
Conversely,
cells that are thin, flattened, hypochromic and misshaped like target cells and
sickle cells have a greater capacity to expand in hypotonic solutions, lyse at
a lower concentration than do normal cells, and are said to have decreased
osmotic fragility.
The
point of beginning hemolysis in each series is noted by looking for the tube
with the highest concentration in which the pink tinting of the supernatant
fluid is detectable.
The
point of complete hemolysis is indicated by the tube in which there are not red
cells left intact.
Methods:
a. Sandord
methods
Normal
values: Initial hemolysis – 0.42 –
0.44%
Complete hemolysis
– 0.32 – 0.34%
b. Dacie’s
Autohemolysis Test – sterile,
defibrinated blood is incubated at 37oC for 48 hours. During this time, red
cells undergo a complex series of change, lose membrane and become more
spherocytic. In normal blood, without added glucose, the amount of
autohemolysis at 48 hours is 0.2 – 2%. In normal blood incubated with added
glucose, the amount of autohemolysis is less, 0 to 0.9%
c. Fragiligraph
method – employs an electronic
instrument. In this method, the blood is allowed to hemolyze in a solution as a
beam of light continuously passes through the solution. The greater the
hemolysis, the greater the transmission of light. Readings are automatically
made at various time intervals, a fragility curve is automatically plotted, and
the results are automatically printed.
d. Micromethod – in this method, insert a dialyzing cell containing
0.075 ml of 1:10 dilution of whole blood in isotonic saline solution, into a
test tube of distilled water. Place the test tube into a colorimeter with a
recorder. The degree of hemolysis is proportional to the increasing transparency
of red cell suspension.
4. Red Cell
Mechanical Fragility Test
Blood
is obtained by venipuncture and then glass or quartz beads are placed in the
same flask containing the blood. Rotate the blood with the beads for 60 minutes
and determine the extent of hemolysis.
Mechanical
fragility test is increased in sickle cell anemia, thalassemia major and
acquired autoimmune hemolytic anemia. It is decreased in spherocytosis.
5. Test for
Paroxysmal Nocturnal Hemoglobinuria
a. Ham’s
Acidified Serum Test
Principle:
The
patient’s red cells are exposed at 37oC to the action of normal or
the patient’s own serum suitably acidified to the optimum pH for lysis (pH 6.5
to 7.0)
b. Sucrose
Hemolysis or Sugar Water Test
Principle:
The
patient’s washed red cells are mixed with ABO compatible normal serum and
isotonic sucrose. The tube is incubated at room temperature for 30 minutes and
then centrifuged, and the percent hemolysis in the supernatant is determined.
c. Crosby’s
Thrombin Test
Principle:
The
patient’s red cells are exposed at 37oC to the action of normal or
the patient’s own serum which has been suitably acidified and which has been
added with commercial preparation of thrombin for lysis.
d. Cobra –
Venom Test
Principle:
The
complement is activated via the alternate pathway by the addition to serum of
partially purified cobra venom. The percentage lysis of PNH red cell sample is
then determined.
e. Heat
Resistance Test – clotted blood is
incubated at 37oC and then inspected for spontaneous lysis.
f. Inulin
Test – a drop of inulin solution is
added to 3 ml freshly collected blood and gently mixed. The mixture is allowed
to stand at 37oC for at least 30 to 45 minutes until the clot is
centrifuged and the supernatant serum is inspected for lysis.
6. Test for
Paroxysmal Cold Hemoglobinuria
a. Qualitative
Donath – Landsteiner Test
Principle
Blood
samples are delivered directly into the test tubes previously warmed in the 37oC
and the other is placed immediately in crushed ice at 0oC and left
undisturbed for 1 hour. The tube is then placed in a water bath at 37oC
without disturbing the clot. Both tubes are examined when the clots have
retracted.
b. Indirect
Antiglobulin Test
Since
the Donath Landsteiner antibody is an IgG antibody, it can be detected by the
indirect antiglobulin test using an anti–IgG serum if the cells are washed in
cold (4oC) buffered 9g/l NaCl.
c. Rosenbach
Test
One
hand or foot of the patient if immersed in ice water for 10 minutes and the
patient’s urine specimens before and after immersion are tested for albumin and
hemoglobin. In PCH, urine darkens in 1 to 6 hours after chilling and the
albumin and hemoglobin tests are positive.
d. Ehrlich’s
Ring Finger Test
This
is done by tightly binding the patient’s finger with a rubber band, immersing
it in ice water and then in warm water, making a finger puncture to obtain
blood, centrifuging the blood and inspecting the serum for hemolysis
7.
Test for
Hemoglobinopathies
a.
Hemoglobin
Electrophoresis
b.
Test for Abnormal
Hemoglobin Pigments
c.
Demonstration of
Heinz bodies
Reagents:
0.5%
Methyl violet in 0.9% sodium
d. Tests for
Unstable Hemoglobin
Heat
Instability Test
Most
unstable hemoglobins precipitate more rapidly than normal hemoglobin when
incubated at 50oC. Both normal and unstable hemoglobin precipitate
more rapidly in Tris buffer than in phosphate buffers. In a hemolysate, an
easily visible precipitate forms within an hour if unstable hemoglobin is
present
Isopropanol
Precipitation Test
A
relatively non–polar solvent weakens the internal bonds of hemoglobin and
decreases its stability. Unstable hemoglobin precipitates within 20 minutes in
the non–polar solvent, isopropanol, whereas a normal hemolysate remains clear
for 30 to 40 minutes
e. Test for fetal
hemoglobin
f. Quantitative
estimation of HbA2
Marengo–Rowe
modified procedure – this is based on the separation of hemoglobin by
electrophoresis on cellulose acetate and subsequent elution of the HbA and HbA2
zones for the measurement of the HbA2 percentage value.
g. Test for
the presence of HbS (Sickling)
Metabisulfite
Microscopic Test
Deoxygenated
cells containing HbS sickle. The process of deoxygenation is enhanced by adding
reducing substances, 2% sodium metabisulfite to the preparation. The slides are
examined under the microscope for presence of “holly leaf” forms and sickled
forms.
Microscopic
Test without a reducing agent
(1)
Scriver
and Waugh method
If
a reducing agent is not available, a drop of blood may be placed on a slide and
a coverglass applied over it and sealed. Sickling will occur after several
hours in a sickle cell anemia; it will take longer in sickle cell trait.
Placing a rubber band around the finger to deoxygenate the blood in vivo before
sampling by finger puncture will shorten the time involved.
(2)
Dithionite
Tube Test or Sickledox Method or Qualitative Solubility Test
HbS
is reduced by dithionite and is insoluble in concentrated inorganic buffers.
The polymers of reduced HbS obstruct light rays from passing through the
solution. Opacity or turbidity indicates insoluble HbS is present.
8. Test for
Glucose–6–phosphate Dehydrogenase (G6PD) Deficiency
a. Demonstration of
Heinz bodies with methyl violet
b. Motulsky’s
Dye Reduction or Brilliant Cresyl Blue Test
This
test is conveniently performed using commercially available kits. In principle,
a mixture of glucose–6–phosphate, NADP and brilliant cresyl blue dye in buffer
is incubated with hemolysate. If G–6–PD is present, the NADP will be reduced to
NADPH, which in turn, will reduce the blue dye to its colorless form.
c. Ascorbate
Cyanide Test by Jacob and Jandl
When
blood is incubated with a solution of sodium cyanide and sodium ascorbate,
hydrogen peroxide is generated from the coupled oxidation of ascorbate and
hemoglobin. Cyanide inhibits catalase, hydrogen peroxide is available to
oxidize hemoglobin, and the brown color of methemoglobin is discernible.
d. Fluorescent
Spot Test by Beutler and Mitchelle
When
blood is added to a mixture of glucose–6–phosphate, NADP, saponin and buffer
and a spot of this mixture is placed on a filter paper and observed for
fluorescence with ultraviolet light. Lack of fluorescence indicate G–6–PD.
e. Quantitative
assay of G–6–PD
9. Test for
Pyruvate Kinase Deficiency
a. Fluorescent
Spot Test by Beutler and Mitchelle
Pyruvate
kinase catalyzes the phosphorylation of ADP to ATP by phosphoenolpyruvate with
the formation of pyruvate. Pyruvate then reduces any NADH present to NAD with
the formation of lactate. Loss of fluorescence of NADH under ultraviolet light
is observed as evidence of the presence of pyruvate kinase.
b. Quantitative Assay of Pyruvate Kinase
10. Coomb’s Test and other tests for incomplete antibodies
11. Test for urobilinogen
12. Test for urinary hemosiderin
13. Test for bilirubin in blood and urine
POLYCYTHEMIA
Polycythemia is an
increased concentration of erythrocytes in the blood that is above the normal
of age and sex. Usually, but not always, the hematocrit and hemoglobin are also
observed.
A. Relative
Polycythemia and Pseudopolycythemia
refers to an increase in hematocrit or red cell count due to decreased plasma
volume; total red cell mass is not increased. This occurs in acute dehydration,
e.g. in severe diarrhea or burns, and in patients on diuretic therapy. A type
of relative polycythemia is stress polycythemia. This is seen in middle – aged
individuals of the aggressive type, usually men who are under stress and
strain. They have a decreased in their total plasma volume and a relative, but
not an absolute increase in their red cell mass. Stress polycythemia is also
called spurious polycythemia.
B. Absolute
Polycythemia refers to an increase in
the total red cell mass in the body. The types of absolute polycythemia:
1. Secondary
Polycythemia
It
can be caused by:
a. Appropriate erythropoietin production due to hypoxia
(1)
Arterial oxygen
unsaturation due to high altitude, pulmonary disease, cyanotic heart disease,
methemoglobinemia, smoker’s polycythemia
(2)
High affinity
hemoglobinopathy
b. Inappropriate erythropoietin production as observed in
(1)
Neoplasms either
benign or malignant
(2)
Renal disorders
c. Familial polycythemia
2. Polycythemia
vera
Synonyms:
True
polycythemia
Erythremia
Primary
polycythemia
Vasquez
– Osler’s disease
Polycythemia
rubra vera
Polycythemia
vera is one of the myeloproliferative
disorders by panmyelosis – excessive proliferation of erythroid, granulocytic
and megakaryocytic elements in the marrow and also in extramedullary sites.
This is reflected in the blood predominantly in an absolute increase in the red
cell mass and also by leukocytosis and thrombocytosis. The cause of this
panmyelosis is unknown.
Laboratory
findings:
a. Red cell count,
leukocyte count and platelet count are increased
b. Increased
hematocrit
c. High LAP or NAP
score
d. Hypercellular
bone marrow and contain little fatty marrow
e. Red cell volume
exceeds 36 ml/kg for men and 32 ml/kg for women
Erythrocytosis
Erythrocytosis is the
special name given to the polycythemia found in association with congenital
heart disease (blue babies), chronic lung disease (emphysema) or other
pulmonary disease leading to considerable reduction in the gas exchange area or
mechanism in the lungs and in those living in high altitudes.
Laboratory findings
1.
Increased
erythrocytes count
2.
Normal white cell
count
3.
Normal platelet
count
BLOOD VOLUME
Measurement of Blood
Volume
Principle
A small volume of readily
identifiable material is injected intravenously and its dilution is measured
after time has been allowed to for the injected material to become thoroughly
mixed in the circulation, but before significant quantities have left the
circulation. Formerly, Evans blue dye was commonly used as the marker. It is
still used occasionally. However, the most practical method now available is to
use a small volume of a patient’s red cell labelled with radioactive chromium (51Cr).
The labelled red cells are diluted in the whole blood of the patient and form
their dilution, the total blood volume and the red cell volume can be
calculated from a knowledge of the packed cell volume or hematocrit.
The most accurate method
of determining the blood volume (BV) is by separate measurement of the plasma
volume (PV) and the erythrocyte volume (EV):
BV = PV + EV
A less accurate method is
by calculation from plasma volume and body hematocrit
BV = 100 x PV
100 –
Body hematocrit
or from the erythrocyte
volume and body hematocrit
BV = 100 x
EV
Body
hematocrit
The body hematocrit is
calculated from the venous hematocrit
Body Hematocrit = venous hematocrit x 0.97 x 0.91
0.97 allows for the trapped plasma remaining in the RBC
column
0.91
allows for the lower RBC content in the blood as a whole than in venous blood
Measurement of
erythrocyte and plasma volume
The diagnosis of absolute
polycythemia depends on reliable measurements of erythrocyte and plasma volume.
The erythrocyte and plasma volume are measured by the use of radioactive
isotope tracers and the dilution principle. The most commonly employed tracers
are 51Cr in the form of sodium chromate bound to erythrocytes for measurement
of erythrocyte volume. Iodine – 125 or Iodine – 131 is bound to albumin and can
be used to measure plasma volume.
Interpretation
The normal erythrocyte volume for men is 20 to 36 ml/kg
or 0.02 to 0.036 liter/kg
For women
is 19 to 31 ml/kg or 0.019 to 0.031 liter/kg
The normal volume for men = 25 to 43 ml/kg or 0.025 to
0.043 liter/kg
For women = 28 to 45 ml/kg or
0.028 to 0.045 liter/kg
The normal total blood volume for men and women = 56 to
76 ml/kg or 0.056 to 0.076 liter/kg

1 comment:
Nice post!
Post a Comment