Types
of specimen needed:
1.
Nasal
or throat swabs or postnatal washing – for respiratory infection
2.
Feces
– for gastrointestinal infection
3.
Vesicle
fluid, throat swab, feces – for vesicular rash
4.
Serum,
feces – for hepatitis
5.
CNS
infection – CSF, throat swab, feces
6.
Unclotted
blood – AIDS
· In
addition, to the above, 5–10 ml of clotted blood for serological tests is
always required.
General
methods of viral diagnosis:
1.
Rapid
diagnostic method
2.
Detection
of antiviral antibodies
3.
Cell
culture
RAPID DIAGNOSTIC METHODS
1.
Monoclonal Antibodies
Mice are immunized with a particular virus;
their spleen cells are then fused with a continuous line of mouse myeloma
cells, which are capable of producing large quantities of antibody. A single
fused cell making antibody of the required specificity is identified and cloned
by limiting dilutions. Since all the spleen B cell produce antibodies of
different specificities, this process is very laborious, but, if successful, a
cell line can be prepared from the single cloned cell that produces large
amounts of antibody of a single specificity indefinitely.
2.
Immunofluorescence Test
a.
Direct Fluorescence Antibody method
Viral antigen is reacted with a specific
antiserum which is coupled with a fluorescein isothiocyanate (FITC). The dye
becomes visible as a green fluorescence viewed by UV microscope.
b.
Indirect Fluorescent Antibody (IFA) method
Antiserum used is unlabeled instead the dye
(usually immunoperoxidase) is attached to a second serum prepared against
globulins from the species in which the specific serum was made which is then
reacted with a substrate to give a precipitate visible by ordinary light
microscopy.
Application
a.
Diagnosis
of RSV in throat washings
b.
Diagnosis
of CMV in a previously inoculated cell culture
3.
Enzyme–linked
Immunosorbent Assay (ELISA) and Radioimmunoassay (RIA)
This has the same principle as with IFA, the
main differences are as follows:
a.
Instead
of a fluorescent dye, the label is either an enzyme (for ELISA) or radioactive
iodine (RIA)
b.
Specific
binding of the labeled antibody (or antigen) is detected by reacting the enzyme
with a substrate which then produces a visible color in the reaction mixture
(ELISA) or by counting radioactive emissions (RIA)
c.
Rather
than on a microscope slide, the reaction takes place in a tube or, more
usually, a multiwell plastic plate, in which the reactions are read by
photometry (for ELISA) or gamma counter (for RIA).
Application:
For identification of viral antigens such as
the p24 antigen of HIV–1, Hepatitis antigen, Rotavirus and their corresponding
antibodies.
4.
Latex agglutination Test
Latex particles are coated with viral antigen
and agglutinate when mixed on a slide with specific antiserum. The test is
rapid, easy to read, and does not require complicated equipment. It is,
however, liable to prozone effects, giving false negative results at low
dilutions of serum.
5.
Electron Microscopy (EM) and Immunoelectron
Microscopy (IEM)
Samples are negatively stained with
phosphotungstic acid, i.e., virions, which are not penetrated by the stain;
stand out as white particles on a dark background. At lease, 106
particles must be present on the EM grid to stand a chance of being identified;
it is sometimes necessary to use concentration method.
Applications:
a.
Rapid
identification of morphologically distinctive virions, usually directly in
clinical specimens, but sometimes in cell culture fluid, HSV and VSV can be
readily identified in vesical fluid, although, being identical in appearance,
they cannot be distinguished from each other by EM.
b.
Identification
of viruses that cannot be grown in cell culture. These include rotaviruses,
adenoviruses and “small round” viruses in feces and HBV in blood.
c.
The
value of the test may on occasion be increased by using IEM, which is the
addition to the specimen of specific immune serum that agglutinates a
particular virus, thus making the virions easier to find and adding serological
specificity to their identification.
6.
Nucleic Acid Hybridization
a.
Dot–blot
hybridization involves extracting the nucleic acid – usually DNA from the
specimen and denaturing it into single strands. Spots of the DNA solution are
placed on a nitrocellulose filter and treated with a probe consisting of a
labeled stretch of DNA or RNA complementary in sequence to the specific region
being sought in the specimen. The label maybe fluorescent
b.
In
situ hybridization is similar to dot–blot, except that the specific nucleic
acid sequences are labeled directly in tissue sections.
Application:
To detect genomes of papillomaviruses and
herpes viruses in tissues and enteric viruses in feces.
7.
Polymerase Chain Reaction
a.
Two
distinct oligonucleotide primer sequences, one on each strand of the target DNA
molecule are added to a clinical sample which has been treated with 94oC
heat and detergent to denature the strands of viral DNA
The primers specifically hybridize with the
homologous nucleotide stretches on the viral DNA genome. A DNA polymerase
called Taq polymerase (from Thermophilus aquaticus), which acts at high
temperature, is added.
b.
After
one minutes, the temperature is reduced to 52oC for 20 seconds to
allow annealing primer.
c.
The
temperature is then raised to 72oC for 5 minutes to allow DNA
polymerization to occur.
d.
DNA’s
can be separated using polyacrylamide gel and visualized by addition to the gel
of ethidium bromide and exposure to UV light.
Application:
To detect HIV proviral DNA, CMV DNA and
Hepatitis B DNA.
DETECTION OF ANTIVIRAL ANTIBODIES
1.
Class specific (IgM) antibody test
IgM is detectable within days of infection
and remains so for 3–9 months, so that its findings are good evidence of a
current or recent infection.
Procedure:
a.
IgM
antibody to human IgM (anti–IgM) is adsorbed to a solid surface, e.g., a well
in a microtiter plate.
b.
The
test serum is then added; IgM molecules are “captured” by the anti – IgM.
c.
Desired
viral antigen is added and attached only to a viral specific IgM.
d.
Enzyme
labeled antibody to a virus is added and detected
Applications:
For rubella virus and reactivation of Herpes
virus.
2.
Western blot method (for HIV)
Procedure
a.
Virus
proteins are separated as bands according to their molecular weights by
electrophoresis through polyacrylamide gel.
b.
The
bands are eluted (blotted) on to chemically treated paper, to which they band
tightly.
c.
The
test serum is added to the paper strip and any specific antibody attached to
the viral proteins.
d.
An
anti–human antibody labeled with an enzyme is added, followed by the enzyme
substrate; the paper is then inspected for the presence of stained bands which
indicate the presence of complexes of specific antibody with antigen.
3.
Complement fixation test (CFT)
Procedure:
a.
The
test serum is reacted with viral antigen and a defined amount of complement.
b.
Specific
antibody, if present, forms a complex with the antigen and complement.
c.
Complement
is then tested for by adding red blood cells sensitized with anti–red cell
antibody. If it is still available, lysis of the red cells will take place
(negative result).
d.
If
however, the complement was previously mopped up by a specific viral
antigen–antibody complex, the red cells are
not lysed by their antibody and sink to the bottom of the wall.
4.
Radial hemolysis test
Procedure:
a.
The
virus is linked to sheep or human red blood cells by chromium chloride.
b.
The
treated cells are mixed with molted agarose which is poured into petri dish or
other suitable plate.
c.
After
cooling, small wells are punched in the agarose, each then being filled with a
serum sample.
d.
The
plate is incubated overnight to allow diffusion of antibody into the agarose
and combination with the antigen on the red cells.
e.
A
solution of complement is poured over the plate, and lysis those red cells on
which both antigen and antibody are present.
f.
Wells
in which the test serum contained antibody are surrounded by clear zones of
lysis, the diameter of which gives an indication of the amount of antibody
present.
Application:
a.
Rubella
antibody
b.
Influenza
antibody
CULTIVATION OF VIRUSES
Importance
of viral culture
1.
For
diagnosis of infection
2.
For
research purposes
3.
For
production of antigens for vaccines and serological agents
Means
of cultivating viruses:
1.
Use
of chick embryo
2.
Use
of laboratory animals
3.
Gene
cloning or recombinant DNA technology
4.
Use
of cell and tissue culture
Types
of culture for viruses:
1.
Cell
cultures – reserved for the propagation
of dispersed cells, either in suspensions or as continuous (confluent) sheets
adhering to glass or plastic surfaces (monolayers).
2.
Tissue
cultures – refers to the growth of
fragments of unorganized tissues, usually fibroblast, in plasma clots.
3.
Organ
cultures – is the maintenance in vitro of
pieces of organized tissue. Viruses growing on an organ (e.g., brain, lung,
intestine, kidney) as substrate for propagation are different to maintain.
Growth medium – used to cultivate cells or tissues,
contains a solution of salts at physiological concentrations, glucose, amino
acids, essential vitamins and antibiotics; it is buffered at pH 7.2 – 7.4.
Fetal calf serum is added to a concentration of 10 – 20% to provide supplements
essential for cell growth.
Maintenance medium – contains 2–5% serum, use for inoculation
for growth medium.
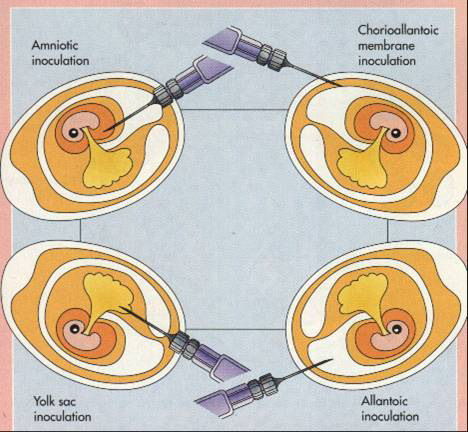
CHICK EMBRYO CULTURE
The time from fertilization of hen’s egg to
hatching is 21 days. Before inoculation, the eggs are incubated at 37oC,
usually for 10–14 days, the exact period depending on the route, which varies
with virus under investigation.
1.
Amniotic
inoculation of 10–11 days old embryos is commonly used to isolate influenza
viruses. A small volume of throat washing is inoculated; the virus if present
replicates the embryonic lung cells and is harvested 2 or 3 days later by
sucking off the amniotic fluid. Antibiotics are added to the specimen to
suppress growth of unwanted bacteria.
2.
Allantoic
inoculation – influenza viruses adapted to chick embryo tissue by growth in the
amnion can be propagated in much larger quantities within the allantoic cavity,
the fluid from which is collected 48 hours after inoculation. This method is
used for vaccine production, harvesting of the allantoic fluid (about 5 ml per
egg) being automated.
3.
Chorioallantoic
membrane – where pox and herpes simplex viruses produce discrete lesions (“pocks”)
1–3 mm in diameter, each of which is focus on cell proliferation generated by
the replication of one virus particle. Pock counts were used to measure the
infectivity of suspensions of these viruses, but the technique has been
superseded by cell culture methods.
CELL AND TISSUE CULTURE
Types
of tissue culture:
1.
Primary culture – for dispersion of cells from host tissue.
Procedure:
a.
Fetal
or adult tissue is collected aseptically and chopped into small pieces of about
2mm3.
b.
Incubation
with trypsin for 30 minutes reduces most of the tissue to a suspension of
individual cells or small clumps.
c.
Centrifuge
to remove excess trypsin.
d.
Suspend
in culture medium
e.
Incubate
at 37oC in screw–capped glass or plastic tubes.
f.
During
the next few days, the cells form a continuous (confluent) layer.
g.
The
culture tubes are often incubated on their sides in a slow roller apparatus,
which exposes the cells alternately to the gas and liquid phased within the
tube.
h.
Such
cultures are at first a mixture of fibroblastic, epithelial and other cells,
but the faster dividing fibroblast tend to outgrow the other.
2.
Semicontinous cell lines or secondary culture – derived from human or animal fetal tissue
and has a diploid karyotype, hence, can be used for vaccine production.
Examples of vaccines produce from
semicontinous culture:
a.
Rabbit
kidney cells – rubella vaccine
b.
Chick
fibroblast – measles vaccine
c.
Monkey
kidney cells – polio vaccine
Sources of semicontinous cell lines:
a.
Human
fetal lung – HDCS, MRC–9 , WI–38
3.
Continuous cell lines – derived from tumor or malignant cells and
are aneuploid (abnormal number of chromosomes) hence cannot be used for vaccine
production.
Sources of continuous cell lines:
a.
HeLa
– Human Cervical carcinoma
b.
Hep–2
– Human epithelium
c.
Vero
– Monkey kidney
d.
MDCK
– dog kidney
Propagation of cell lines:
a.
Monolayers
A monolayer in a culture flask is treated
with trypsin or versene to dispose it into a suspension of individual cells,
which are then diluted in growth medium to a concentration of 105 to
106 per ml and distributed into other flask, tubes or petri dishes
for further subculture.
Usually, for virus isolation, small stoppered
test tubes are used, incubated at 37oC at a slight slope. Within an hour, the
cells attach to the side of the tube and begin to divide, to give a confluent
monolayer by 48 hours. The growth medium is then substituted by maintenance
medium and the tubes are inoculated with a small volume of the clinical
specimen, e.g., throat washing, stool suspension or vesicle fluid.
b.
Mass cultures
Use for propagation of large quantities of
virus, e.g., for vaccine production. This is accomplish by a continuous
suspension in fermenters or allowing it to grow in surfaces (like Sephadex
beads)
EXPECTED REACTION OF VIRUSES ON
CULTURE MEDIA
1.
Cytopathic Effect (CPE) – these are changes in cell morphology and
give information of the type of virus isolated though it must be confirmed by
other tests.
Types of CPE:
a.
“Burster”
viruses (e.g. enterovirus) – causes rounding up and lysis
b.
“Creeper”
viruses – formation of multinucleate giant cells (syncytia), with or without
“ballooning” of clumps of cells.
e.g. Herpesvirus, Paramyxoviruses
A few viruses, although replicating in the
cell culture, cause no visible CPE and are detected only by their ability to
make the cells resistant to superinfection with a second virus. Other viruses
not causing CPE can be detected by immunofluorescence or haemadsorption.
For isolating HIV–1 from AIDS patients, a
special technique had to be developed, since this agent grows only in human
lymphocytes, which cannot normally maintained in culture. This difficulty was
overcome by stimulating them with a plant lectin, phytohemagglutinin and
interleukin–2.
2.
Plaque formation
Plaques are circular cleared areas within a
monolayer. These are exactly analogues to those caused lawns of bacteria by
bacteriophages and, like them, can be counted to estimate the number of infective
viruses in the original suspensions. Plaques caused by both bacterial and
animal viruses have another important use, this time in genetic studies, in
which those generated by mutated strains can be recognized by alterations in
their appearance.
Laboratory
Equipment:
1.
35oC
aerobic incubator
2.
Two
or three roller drums with motordriven bases to rotate the drums
3.
Inverted
microscope to examine cell culture flasks and tubes
4.
Laminar
flow hood – to carry out cell culture procedures
5.
Conventional
horizontal head centrifuge
6.
Refrigerator
(4oC) with –20oC
freezer for reagents and media storage
7.
Fluorescent
microscope
8.
Ultracold
freezing unit (–70oC) to store reagents, antigens and other
materials.
Selection
of specimens for viral culture
1.
Proper
timing of specimen collection is essential
2.
Collected
early in the acute phase of infection
e.g. Respiratory
viruses – 3–7 days
HSV
and VZV – may not be recovered beyond 5 days after onset
Enterovirus
– within 2 to 3 days after onset of the CNS manifestations
Transport
of specimens to the laboratory
1.
Do
not freeze specimens. Obtain specimens and inoculate tissue cultures at
patient’s bedside if possible (except blood, feces and tissues)
2.
Any
material may be used for swabs (calcium alginate may inactivate HSV)
3.
Never
leave a specimen at room temperature or incubator temperature
4.
When
it is impossible to deliver a specimen immediately, should be refrigerated and
packed in shaved ice for delivery within 12 hours of collection.
5.
Transport
media
a.
Modified
Stuart’s
b.
Hank’s
– protein (serum, albumin, gelatin) incorporated into a transport medium
enhances the survival of viruses in transit.
c.
Leibovitz–Emory
media
d.
Culturette
(modified Stuart’s Bacterial Transport Medium, Marion Lab) – satisfactory for
short term (i.e., up to 4 hours) transport.
6.
Improper
storage can significantly reduce viral culture yields [short term (<5 days)
transit or storage of specimens for viral culture, specimen should be held at 4oC
rather than frozen]
7.
The
specimen should be wrapped in sufficient absorbent material to absorb the
entire contents of the specimen in case of leakage or breakage
8.
The
label “Etiologic Agent” or “Biomedical Material” must appear on the outside of
the shipping container




No comments:
Post a Comment